

新型吡唑酰基硫脲类化合物的设计合成、杀菌活性与分子对接研究
收稿日期: 2017-04-18
修回日期: 2017-06-02
网络出版日期: 2017-06-16
基金资助
浙江省自然科学基金(No.LY16C14007)和国家自然科学基金(No.31401691)资助项目.
Design,Synthesis,Fungicidal Activity and Docking Study of Acyl Thiourea Derivatives Containing Pyrazole Moiety
Received date: 2017-04-18
Revised date: 2017-06-02
Online published: 2017-06-16
Supported by
Project supported by the Natural Science Foundation of Zhejiang Province (No.LY16C14007) and the National Natural Science Foundation of China (No.31401691).
孙娜波 , 沈钟华 , 翟志文 , 韩亮 , 翁建全 , 谭成侠 , 刘幸海 . 新型吡唑酰基硫脲类化合物的设计合成、杀菌活性与分子对接研究[J]. 有机化学, 2017 , 37(10) : 2705 -2710 . DOI: 10.6023/cjoc201704032
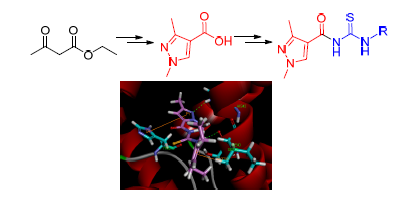
A series of novel acyl thiourea derivatives containing pyrazole moiety were designed and synthesized from ethyl acetoacetate, triethyl orthoformate, methylhydrazine by multi-step reactions. Their structures were characterized by 1H NMR, 13C NMR and HRMS. The target compounds were evaluated for their fungicidal activity. The results indicated that some of the title compounds displayed certain fungicidal activities against Botryospuaeria berengeriana. The docking results indicated that the hydrogen bond and π(σ)-π interaction formed between N-((2,6-diethylphenyl)carbamothioyl)-1,3-dimethyl-1H-pyrazole-4-carboxamide (6k) and succinodehydrogenase.
Key words: pyrazole; acyl thiourea; synthesis; fungicidal activity; docking
[1] Russell, P E. Outlooks Pest Manage. 2009, 20, 122.
[2] Mu, J. X.; Shi, Y. X.; Yang, M. Y.; Sun, Z. H.; Liu, X. H.; Li, B. J.; Sun, N. B. Molecules 2016, 21, 68.
[3] Elbe, H.-L.; Rieck, H.; Dunkel, R.; Wachendorff-Neumann, U.; Mauler-Machnik, A.; Kuck, K.-H.; Kugler, M.; Jaetsch, T. WO 2002008195, 2002[Chem. Abstr. 2002, 136, 151162].
[4] Elbe, H.-L.; Rieck, H.; Dunkel, R.; Zhu-Ohlbach, Q.; Mauler-Machnik, A.; Wachendorff-Neumann, U.; Kuck, K.-H. WO 2003010149, 2003[Chem. Abstr. 2003, 136, 137308].
[5] Du, S.; Tian, Z.; Yang, D.; Li, X.; Li, H.; Jia, C.; Che, C.; Wang, M.; Qin, Z. Molecules 2015, 20, 8395.
[6] Xiong, L.; Zhu, X. L.; Shen, Y. Q.; Wishwajith, W. K. W. M.; Li, K.; Yang, G. F. Eur. J. Med. Chem. 2015, 95, 424.
[7] Xiong, L.; Zhu, X. L.; Gao, H. W.; Fu, Y.; Hu, S. Q.; Jiang, L. N.; Yang, W. C; Yang, G. F. J. Agric. Food Chem. 2015, 64, 4830.
[8] Liu, X. H.; Zhao, W.; Shen, Z. H.; Xing, J. H.; Yuan, J.; Yang, G.; Xu, T. M.; Peng, W. L. Bioorg. Med. Chem. Lett. 2016, 26, 3626.
[9] Zhao, W.; Xing, J. H.; Xu, T. M.; Peng, W. L.; Liu, X. H. Front. Chem. Sci. Eng. 2017, 11, 363.
[10] Zhao, W.; Shen, Z. H.; Xu, T. M.; Peng, W. L.; Liu, X. H. Lett. Drug Des. Discovery 2017, 14, 323.
[11] Zhao, W.; Shen, Z. H.; Xing, J. H.; Yang, G.; Xu, T. M.; Peng, W. L.; Liu, X. H. Chem. Pap. 2017, 71, 921.
[12] Zhao, W.; Shen, Z. H.; Xing, J. H.; Xu, T. M.; Peng, W. L.; Liu, X. H. Chin. J. Struct. Chem. 2017, 36, 423.
[13] Liu, X. H.; Tan, C. X.; Weng, J. Q. Phosphorus, Sulfur Silicon Relat. Elem. 2011, 186, 558.
[14] Zhai, Z. W.; Wang, Q.; Shen, Z. H.; Tan, C. X.; Weng, J. Q.; Liu, X. H. Chin. J. Org. Chem. 2017, 37, 232(in Chinese) (翟志文, 汪乔, 沈钟华, 谭成侠, 翁建全, 刘幸海, 有机化学, 2017, 37, 232.)
[15] Chen, W.; Wei, W.; Wu, C. C.; Li, Y. X.; Li, Y. H.; Yu, S. J.; Li, Z. M. Chin. J. Org. Chem. 2015, 35, 1576(in Chinese). (陈伟, 魏巍, 吴长春, 李玉新, 李永红, 于淑晶, 李正名, 有机化学, 2015, 35, 1576.)
[16] Chen, Y. W.; Wan, Y. Y.; Liu, Q. X.; Liu, J. B.; Xiong, L. X.; Yu, S. J.; Li, Z. M. Chin. J. Org. Chem. 2015, 35, 882(in Chinese) (陈有为, 万莹莹, 刘巧霞, 刘敬波, 熊丽霞, 于淑晶, 李正名, 有机化学, 2015, 35, 882.)
[17] Li, C. K.; Jiang, L.; Wang, Y.; Wan, F. X.; Zhang, P. Z.; Li, Y.; Cui, Z. N. Chin. J. Org. Chem. 2014, 34, 2296(in Chinese) (李成坤, 姜林, 王悦, 万福贤, 张沛之, 李映, 崔紫宁, 有机化学, 2014, 34, 2296.)
[18] Liu, X. H.; Wang, Q.; Sun, Z. H.; Wedge, D. E.; Becnel, J. J.; Estep, A. S.; Tan, C. X.; Weng, J. Q. Pest Manage. Sci. 2017, 73, 953.
[19] Chen, J. N.; Wang, X. F.; Li, T.; Wu, D. W.; Fu, X. B.; Zhang, G. J.; Shen, X. C.; Wang, H. S. Eur. J. Med. Chem. 2016, 107, 12.
[20] Liu, X. H.; Zhai, Z. W.; Xu, X. Y.; Yang, M. Y.; Sun, Z. H.; Weng, J. Q.; Tan, C. X.; Chen, J. Bioorg. Med. Chem. Lett. 2015, 25, 5524.
[21] Zhai, Z. W.; Shi, Y. X.; Yang, M. Y.; Zhao, W.; Sun, Z. H.; Weng, J. Q.; Tan, C. X.; Liu, X. H.; Li, B. J.; Zhang, Y. G. Lett. Drug Des. Discovery 2016, 13, 521.
[22] Liu, X. H.; Tan, C. X.; Weng, J. Q. Phosphorus, Sulfur Silicon Relat. Elem. 2011, 186, 552.
[23] Zhang, L. J.; Yang, M. Y.; Sun, Z. H.; Tan, C. X.; Weng, J. Q.; Wu, H. K.; Liu, X. H. Lett. Drug Des. Discovery 2014, 11, 1107.
[24] Liu, X. H.; Fang, Y. M.; Xie, F.; Zhang, R. R.; Shen, Z. H.; Tan, C. X.; Weng, J. Q.; Xu, T. M.; Huang, H. Y. Pest Manage. Sci. 2017, 73, 1900.
[25] Yan, S. L.; Yang, M. Y.; Sun, Z. H.; Min, L. J.; Tan, C. X.; Weng, J. Q.; Wu, H. K.; Liu, X. H. Lett. Drug Des. Discovery 2014, 11, 940.
[26] Sun, N. B.; Shen, Z. H.; Zhai, Z. W.; Wu, H. K.; Weng, J. Q.; Tan, C. X.; Liu, X. H. Chin. J. Org. Chem. 2017, 37, 2044(in Chi-nese). (孙娜波, 沈钟华, 翟志文, 武宏科, 翁建全, 谭成侠, 刘幸海, 有机化学, 2017, 37, 2044.)
[27] Zhao, W.; Shen, Z. H.; Xu, T. M.; Peng, W. L.; Liu, X.-H. J. Heterocycl. Chem. 2017, 54, 1751.
[28] Liu, X. H.; Zhao, W.; Shen, Z. H.; Xing, J. H.; Xu, T. M.; Peng, W. L. Eur. J. Med. Chem. 2017, 125, 881.
/
| 〈 |
|
〉 |