

应用于构建优势杂环骨架的交叉脱氢偶联反应
收稿日期: 2017-03-20
修回日期: 2017-06-14
网络出版日期: 2017-07-07
基金资助
国家自然科学基金(Nos.81325020,81761128022)资助项目.
Cross-Dehydrogenative Coupling Reactions Applied in the Construction of Privileged Heterocycles
Received date: 2017-03-20
Revised date: 2017-06-14
Online published: 2017-07-07
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 81325020, 81761128022).
胡玮 , 龙亚秋 . 应用于构建优势杂环骨架的交叉脱氢偶联反应[J]. 有机化学, 2017 , 37(11) : 2850 -2858 . DOI: 10.6023/cjoc201703033
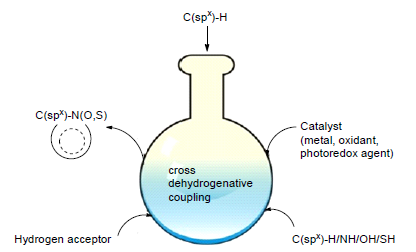
The reactions in which a new C-C bond is formed via a direct coupling of two C-H bonds are termed as cross dehydrogenative coupling (CDC). The coupling reactions would not need the pre-activation of the inert C-H bonds by any func-tional groups, featured with straightforwardness, atom-and step-economy and environmently benigness. Heterocycles are widely found in bioactive natural products and pharmaceuticals, designated as drug-like privileged scaffolds since they endowed diverse pharmacological effects. Preparation of these privileged structures by means of CDC confers distinctive advantages and enhances the discovery of lead compounds in medicinal chemistry. The application of CDC reactions in the synthesis of the heterocycles of pharmaceutical interest in recent years is summarized, focused on the construction of privileged scaffolds, such as indole, pyrrole, quinazoline, quinoxaline and the middle size and poly ring systems.
[1] (a) Wang, M.; Wang, Z.; Shang, M.; Dai, H. Chin. J. Org. Chem. 2015, 35, 570(in Chinese). (王明明, 王子潇, 商明, 戴辉雄, 有机化学, 2015, 35, 570.)
(b) Lu, B.; Li, X.; Lin, Y. Chin. J. Org. Chem. 2015, 35, 2275(in Chinese). (卢贝丽, 李现艳, 林咏梅, 有机化学, 2015, 35, 2275.)
(c) Ding, Z.; Tan, Q.; Liu, B.; Zhang, K.; Xu, B. Acta Chim. Sinica 2015, 73, 1302(in Chinese). (丁正伟, 谭启涛, 刘秉新, 张可, 许斌, 化学学报, 2015, 73, 1302.)
(d) Yu, J.-Q.; Ding, K.-L. Acta Chim. Sinica 2015, 73, 1223(in Chinese). (余金权, 丁奎岭, 化学学报, 2015, 73, 1223.)
[2] (a) Girard, S. A.; Knauber, T.; Li, C.-J. Angew. Chem., Int. Ed. 2014, 53, 74.
(b) Li, G.; Nakamura, H. Angew. Chem., Int. Ed. 2016, 55, 6758.
[3] (a) Li, Z.; Li, C.-J. J. Am. Chem. Soc. 2005, 127, 6968.
(b) Li, Z.; Li, C.-J. J. Am. Chem. Soc. 2006, 128, 56.
(c) Zhang, Y.; Li, C.-J. J. Am. Chem. Soc 2006, 128, 4242.
[4] (a) Zhang, C.; Jiao, N. Angew. Chem., Int. Ed. 2010, 49, 6174.
(b) Han, W.; Mayer, P.; Ofial, A. R. Angew. Chem., Int. Ed. 2011, 50, 2178.
(c) Antonchick, A. P.; Burgmann, L. Angew. Chem., Int. Ed. 2013, 52, 3267.
(d) Meng, Z.; Sun, S.; Yuan, H.; Lou, H.; Liu, L. Angew. Chem., Int. Ed. 2014, 53, 543.
(e) Zhou, L.; Xu, B.; Zhang, J. Angew. Chem., Int. Ed. 2015, 54, 9092.
[5] Gensch, T.; Klauck, F. J. R.; Glorius, F. Angew. Chem., Int. Ed. 2016, 55, 11287.
[6] Lin, J.-P.; Zhang, F.-H.; Long, Y.-Q. Org. Lett. 2014, 16, 2822.
[7] Hu, W.; Lin, J.-P.; Song, L.-R.; Long, Y.-Q. Org. Lett. 2015, 17, 1268.
[8] Zhao, M.; Wang, F.; Li, X. Org. Lett. 2012, 14, 1412.
[9] Vanjari, R.; Guntreddi, T.; Kumar, S.; Singh, K. N. Chem. Commun. 2015, 51, 366.
[10] (a) Wang, L.; Woods, K. W.; Li, Q.; Barr, K. J.; McCroskey, R. W.; Hannick, S. M.; Gherke, L.; Credo, R. B.; Hui, Y.-H.; Marsh, K.; Warner, R.; Lee, J. Y.; Zielinski-Mozng, N.; Frost, D.; Rosenberg, S. H.; Sham, H. L. J. Med. Chem. 2002, 45, 1697.
(b) Zeng, L.-F.; Wang, Y.; Kazemi, R.; Xu, S.; Xu, Z.-L.; Sanchez, T. W.; Yang, L.-M.; Debnath, B.; Odde, S.; Xie, H.; Zheng, Y.-T.; Ding, J.; Neamati, N.; Long, Y.-Q. J. Med. Chem 2012, 55, 9492.
(c) Lam, T.; Hilgers, M. T.; Cunningham, M. L.; Kwan, B. P.; Nelson, K. J.; Brown-Driver, V.; Ong, V.; Trzoss, M.; Hough, G.; Shaw, K. J.; Finn, J. J. Med. Chem. 2014, 57, 651.
[11] Li, C.-J. Acc. Chem. Res. 2009, 42, 335.
[12] Xue, D.; Long, Y.-Q. J. Org. Chem. 2014, 79, 4727.
[13] Zhu, C.; Yang, B.; Qiu, Y.; Backvall, J. E. Angew. Chem., Int. Ed. 2016, 55, 14405.
[14] (a) Cheng, Y.; Shen, J.; Peng, R.-Z.; Wang, G.-F.; Zuo, J.-P.; Long, Y.-Q. Bioorg. Med. Chem. Lett. 2016, 26, 2900.
(b) Zhi, Y.; Gao, L.-X.; Jin, Y.; Tang, C.-L.; Li, J.-Y.; Li, J.; Long, Y.-Q. Bioorg. Med. Chem. 2014, 22, 3670.
[15] (a) Shi, Z.; Glorius, F. Angew. Chem., Int. Ed. 2012, 51, 9220.
(b) Li, J.; Li, C.; Yang, S.; An, Y.; Wu, W.; Jiang, H. J. Org. Chem. 2016, 81, 7771.
(c) Gao, S.; Wu, Z.; Fang, X.; Lin, A.; Yao, H. Org. Lett. 2016, 18, 3906.
(d) Chen, J.; Wu, J. Angew. Chem., Int. Ed. 2017, 56, 3951.
(e) Li, J.; Li, C.; Yang, S.; An, Y.; Wu, W.; Jiang, H. J. Org. Chem. 2016, 81, 2875.
[16] Würtz, S.; Rakshit, S.; Neumann, J. J.; Dröge, T.; Glorius, F. Angew. Chem., Int. Ed. 2008, 120, 7340.
[17] Shi, Z.; Zhang, C.; Li, S.; Pan, D.; Ding, S.; Cui, Y.; Jiao, N. Angew. Chem., Int. Ed. 2009, 48, 4572.
[18] Yu, W.; Du, Y.; Zhao, K. Org. Lett. 2009, 11, 2417.
[19] Guan, Z.-H.; Yan, Z.-Y.; Ren, Z.-H.; Liu, X.-Y.; Liang, Y.-M. Chem. Commun. 2010, 46, 2823.
[20] Zoller, J.; Fabry, D. C.; Ronge, M. A.; Rueping, M. Angew. Chem., Int. Ed. 2014, 53, 13264.
[21] Wei, Y.; Deb, I.; Yoshikai, N. J. Am. Chem. Soc. 2012, 134, 9098.
[22] Jiang, L.; Jin, W.; Hu, W. ACS Catal. 2016, 6, 6146.
[23] Tanitame, A.; Oyamada, Y.; Ofuji, K.; Fujimoto, M.; Iwai, N.; Hiyama, Y.; Suzuki, K.; Ito, H.; Terauchi, H.; Kawasaki, M.; Nagai, K.; Wachi, M.; Yamagishi, J.-I. J. Med. Chem. 2004, 47, 3693.
[24] Wu, C.-H.; Hung, M.-S.; Song, J.-S.; Yeh, T.-K.; Chou, M.-C.; Chu, C.-M.; Jan, J.-J.; Hsieh, M.-T.; Tseng, S.-L.; Chang, C.-P.; Hsieh, W.-P.; Lin, Y.; Yeh, Y.-N.; Chung, W.-L.; Kuo, C.-W.; Lin, C.-Y.; Shy, H.-S.; Chao, Y.-S.; Shia, K.-S. J. Med. Chem. 2009, 52, 4496.
[25] Fustero, S.; Sánchez-Roselló, M.; Barrio, P.; Simón-Fuentes, A. Chem. Rev. 2011, 111, 6984.
[26] Neumann, J. J.; Suri, M.; Glorius, F. Angew. Chem., Int. Ed. 2010, 49, 7790.
[27] (a) Zhang, G.; Zhao, Y.; Ge, H. Angew. Chem., Int. Ed. 2013, 52, 2559.
(b) Wu, X.; Wang, M.; Zhang, G.; Zhao, Y.; Wang, J.; Ge, H. Chem. Sci. 2015, 6, 5882.
[28] Murarka, S.; Studer, A. Org. Lett. 2011, 13, 2746.
[29] Ye, J. T.; Ma, S. M. Acc. Chem. Res. 2014, 47, 989.
[30] Xiao, T.; Li, L.; Lin, G.; Mao, Z.-W.; Zhou, L. Org. Lett. 2014, 16, 4232.
[31] Zhang, Z.; Dai, Z.; Ma, X.; Liu, Y.; Ma, X.; Li, W.; Ma, C. Org. Chem. Front. 2016, 3, 799.
[32] Ackermann, L.; Pospech, J. Org. Lett. 2011, 13, 4153.
[33] Zhang, Z.; Xie, C.; Tan, X.; Song, G.; Wen, L.; Gao, H.; Ma, C. Org. Chem. Front. 2015, 2, 942.
[34] Sun, M.; Zhang, T.; Bao, W. J. Org. Chem. 2013, 78, 8155.
[35] (a) Dhiman, S.; Mishra, U. K.; Ramasastry, S. S. V. Angew. Chem., Int. Ed. 2016, 55, 7737.
(b) Garkhedkar, A. M.; Senadi, G. C.; Wang, J. J. Org. Lett. 2017, 19, 488.
(c) Cheng, J.; Li, W. P.; Duan, Y. Q.; Cheng, Y. X.; Yu, S. Y.; Zhu, C. J. Org. Lett. 2017, 19, 214.
[36] Gaster, E.; Vainer, Y.; Regev, A.; Narute, S.; Sudheendran, K.; Werbeloff, A.; Shalit, H.; Pappo, D. Angew. Chem., Int. Ed. 2015, 54, 4198.
/
| 〈 |
|
〉 |