

钯催化取代吲哚与苯丙酮类化合物的直接偶联构筑咔唑衍生物
收稿日期: 2017-05-08
修回日期: 2017-07-06
网络出版日期: 2017-08-11
基金资助
国家自然科学基金(Nos.21431008,21332001,21602221,u1505242)资助项目.
Construction of Carbazoles by Palladium-Catalyzed Direct Cross-Coupling of Indoles with in situ Generated Aryl Vinyl Ketones
Received date: 2017-05-08
Revised date: 2017-07-06
Online published: 2017-08-11
Supported by
Project supported by the National Natural Science Foundation of China (Nos.21431008,21332001,21602221,u1505242).
周全龙 , 朱昌垒 , 吴戈 , 张远飞 , 张敏 , 苏伟平 . 钯催化取代吲哚与苯丙酮类化合物的直接偶联构筑咔唑衍生物[J]. 有机化学, 2017 , 37(10) : 2655 -2662 . DOI: 10.6023/cjoc201705014
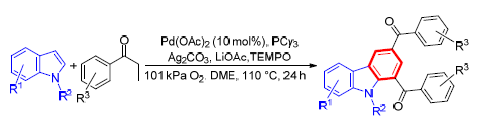
The synthesis of carbazoles via Pd-catalyzed direct cross-coupling of indoles with in situ generated aryl vinyl ketones by using statured ketones as the olefins source is described. This protocol obviates the need for additional preparation steps of aryl vinyl ketones and therefore opens up a new door to synthesis of carbazoles in an atom-and step-economical fashion.
[1] (a) Zhang, F.-F.; Gan, L.-L.; Zhou, C.-H. Bioorg. Med. Chem. Lett. 2010, 20, 1881.
(b) Blunt, J. W.; Copp, B. R.; Munro, M. H. G.; Northcote, P. T.; Prinsep, M. R. Nat. Prod. Rep. 2003, 20, 1.
(c) Deslandes, S.; Chassaing, S.; Delfourne, E. Mar. Drugs 2009, 7, 754.
(d) Maneerat, W.; Ritthiwigrom, T.; Cheenpracha, S.; Promgool, T.; Yossathera, K.; Deachathai, S.; Phakhodee, W.; Laphookhieo, S. J. Nat. Prod. 2012, 75, 741.
[2] (a) Roy, J.; Jana, A. K.; Mal, D. Tetrahedron 2012, 68, 6099.
(b) Knölker, H.-J.; Reddy, K. R. Chem. Rev. 2002, 102, 4303.
(c) Schmidt, A. W.; Reddy, K. R.; Knölker, H.-J. Chem. Rev. 2012, 112, 3193.
[3] (a) Ackermann, L.; Vicente, R.; Kapdi, A. R. Angew. Chem., Int. Ed. 2009, 48, 9792.
(b) Lyons, T. W.; Sanford, M. S. Chem. Rev. 2010, 110, 1147.
(c) Liu, C.; Zhang, H.; Shi, W.; Lei, A. Chem. Rev. 2011, 111, 1780.
(d) Yeung, C. S.; Dong, V. M. Chem. Rev. 2011, 111, 1215.
[4] Zhao, J.; Larock, R. C. J. Org. Chem. 2006, 71, 5340.
[5] (a) Tsang, W. C. P.; Zheng, N.; Buchwald, S. L. J. Am. Chem. Soc. 2005, 127, 14560.
(b) Jordan-Hore, J. A.; Johansson, C. C.; Beck, E. M.; Gaunt, M. J. J. Am. Chem. Soc. 2008, 130, 16184.
(c) Cho, S. H.; Yoon, J.; Chang, S. J. Am. Chem. Soc. 2011, 133, 5996.
(d) Takamatsu, K.; Hirano, K.; Satoh, T.; Miura, M. Org. Lett. 2014, 16, 2892.
[6] (a) Yamashita, M.; Horiguchi, H.; Hirano, K.; Satoh, T.; Miura, M. J. Org. Chem. 2009, 74, 7481.
(b) Jia, J.; Shi, J.; Zhou, J.; Liu, X.; Song, Y.; Xu, H. E.; Yi, W. Chem. Commun. 2015, 51, 2925.
[7] (a) Ozaki, K.; Zhang, H.; Ito, H.; Lei, A.; Itami, K. Chem. Sci. 2013, 4, 3416.
(b) Verma, A. K.; Danodia, A. K.; Saunthwal, R. K.; Patel, M.; Choudhary, D. Org. Lett. 2015, 17, 3658.
(c) Laha, J. K.; Dayal, N. Org. Lett 2015, 17, 4742.
(d) Chen, S.; Li, Y.; Ni, P.; Huang, H.; Deng, G. J. Org. Lett 2016.
[8] Grimster, N. P.; Gauntlett, C.; Godfrey, C. R.; Gaunt, M. J. Angew. Chem., Int. Ed. 2005, 44, 3125.
[9] Guo, T.; Jiang, Q.; Huang, F.; Chen, J.; Yu, Z. Org. Chem. Front. 2014, 1, 707.
[10] (a) Muzart, J. Eur. J. Org. Chem. 2010, 3779.
(b) Newhouse, T.; Turlik, A.; Chen, Y. Synlett 2016, 27, 331.
[11] (a) Nicolaou, K. C.; Gray, D. L. F.; Montagnon, T.; Harrison, S. T. Angew. Chem., Int. Ed. 2002, 41, 996.
(b) Nicolaou, K. C.; Montagnon, T.; Baran, P. S. Angew. Chem., Int. Ed. 2002, 41, 993.
(c) Nicolaou, K. C.; Montagnon, T.; Baran, P. S.; Zhong, Y. L. J. Am. Chem. Soc. 2002, 124, 2245.
(d) Uyanik, M.; Akakura, M.; Ishihara, K. J. Am. Chem. Soc. 2009, 131, 251.
(e) Nicolaou, K. C.; Zhong, Y. L.; Baran, P. S. J. Am. Chem. Soc. 2000, 122, 7596.
[12] (a) Bhattacharya, A.; DiMichele, L. M.; Dolling, U. H.; Douglas, A. W.; Grabowski, E. J. J. J. Am. Chem. Soc. 1988, 110, 3318.
(b) Walker, D.; Hiebert, J. D. Chem. Rev. 1967, 67, 153.
[13] (a) Diao, T.; Stahl, S. S. J. Am. Chem. Soc. 2011, 133, 14566.
(b) Gao, W. M.; He, Z. Q.; Qian, Y.; Zhao, J.; Huang, Y. Chem. Sci. 2012, 3, 883.
(c) Diao, T.; Wadzinski, T. J.; Stahl, S. S. Chem. Sci. 2012, 3, 887.
(d) Diao, T.; Pun, D.; Stahl, S. S. J. Am. Chem. Soc. 2013, 135, 8205.
(e) Bigi, M. A.; White, M. C. J. Am. Chem. Soc. 2013, 135, 7831.
[14] (a) Zhang, M.; Zhang, Y.; Jie, X.; Zhao, H.; Li, G.; Su, W. Org. Chem. Front. 2014, 1, 843.
(b) Wei, Y.; Hu, P.; Zhang, M.; Su, W. Chem. Rev. 2017.
[15] (a) Zhang, M.; Zhou, J.; Kan, J.; Wang, M.; Su, W.; Hong, M. Chem. Commun. 2010, 46, 5455.
(b) Zhou, J.; Wu, G.; Zhang, M.; Jie, X.; Su, W. Chem.-Eur. J. 2012, 18, 8032.
(c) Zhang, M.; Hu, P.; Zhou, J.; Wu, G.; Huang, S.; Su, W. Org. Lett. 2013, 15, 1718.
(d) Shang, Y.; Jie, X.; Zhou, J.; Hu, P.; Huang, S.; Su, W. Angew. Chem. Int. Ed. 2013, 52, 1299.
[16] Jie, X.; Shang, Y.; Zhang, X.; Su, W. J. Am. Chem. Soc. 2016, 138, 5623.
[17] Xiao, B.; Li, Y. M.; Liu, Z. J.; Yang, H. Y.; Fu, Y. Chem. Commun. 2012, 48, 4854.
[18] Klare, H. F.; Oestreich, M.; Ito, J.; Nishiyama, H.; Ohki, Y.; Tatsumi, K. J. Am. Chem. Soc. 2011, 133, 3312.
[19] Taylor, J. E.; Jones, M. D.; Williams, J. M. J.; Bull, S. D. Org. Lett. 2010, 12, 5740.
[20] Qi, T.; Qiu, W.; Liu, Y.; Zhang, H.; Gao, X.; Liu, Y.; Lu, K.; Du, C.; Yu, G.; Zhu, D. J. Org. Chem. 2008, 73, 4638.
/
| 〈 |
|
〉 |