

铜催化对C-C不饱和键铜氢化引发的多米诺反应
收稿日期: 2017-06-02
修回日期: 2017-07-04
网络出版日期: 2017-08-16
基金资助
辽宁省自然科学基金(No.2015020678)资助项目.
Domino Reactions Initialized by Copper-Catalyzed Hydrocupration of C-C Unsaturated Bonds
Received date: 2017-06-02
Revised date: 2017-07-04
Online published: 2017-08-16
Supported by
Project supported by the Natural Science Foundation of Liaoning Province (No. 2015020678).
梁婷婷 , 姜岚 , 干苗苗 , 苏鑫 , 李争宁 . 铜催化对C-C不饱和键铜氢化引发的多米诺反应[J]. 有机化学, 2017 , 37(12) : 3096 -3111 . DOI: 10.6023/cjoc201706004
Compared with sequential reactions, domino reactions are more efficient and highly desirable in organic synthesis as fewer operational and purification procedures are involved, yielding the product with complexity in a more economic and environmently friendly manner. Domino reactions induced by copper-catalyzed hydrocupration of unsaturated C-C bonds in α,β-unsaturated ketones, α,β-unsaturated carboxylates, aryl alkenes, aliphatic alkenes and even alkynes are reviewed. A hydrosilane is used as a hydride source for CuH formation and the hydrocupration intermediates undergo subsequent addition to polar unsaturated bonds, e.g. carbonyls and imines, or proceed to substitution reactions, and finally, reaction involving two or more newly formed bonds proceed without purification of intermediate products or changing the operational conditions. Because of its simplicity and efficiency, this method is highly valuable in organic synthesis.
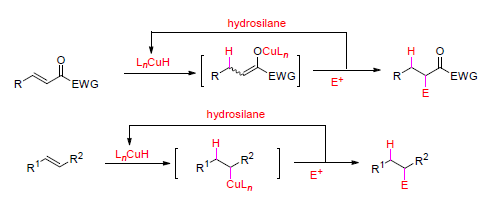
Key words: domino reaction; catalysis; copper; hydrocupration; reduction; alkene
[1] Tietze, L. F. Chem. Rev. 1996, 96, 115.
[2] Pellissier, H. Chem. Rev. 2013, 113, 442.
[3] Saebang, Y.; Rukachaisirikul, V.; Kaeobamrung, J. Tetrahedron Lett. 2017, 58, 168.
[4] Pellissier, H. Adv. Synth. Catal. 2015, 357, 2745.
[5] Li, X.-W.; Wang, C.-Y.; Zheng, L.-Y. Chin. J. Org. Chem. 2006, 26, 1144(in Chinese). (李雄武, 汪朝阳, 郑绿茵, 有机化学, 2006, 26, 1144.)
[6] Chen, J.; Liu, Q.; Dai, X.-Y.; Nie, L.-L.; Fang, H.-H.; Wu, X.-Y. Chin. J. Org. Chem. 2013, 33, 1(in Chinese). (陈杰, 刘钦, 戴小鸯, 聂凛凛, 房辉辉, 吴小余, 有机化学, 2013, 33, 1.)
[7] Zhu, H.; Ye, C.-Q.; Chen, Z.-Y. Chin. J. Org. Chem. 2015, 35, 2291(in Chinese). (朱辉, 叶长青, 陈知远, 有机化学, 2015, 35, 2291.)
[8] Feng, Y.-D.; Zhang, H.; Cheng, G.-L.; Cui, X.-L. Chin. J. Org. Chem. 2014, 34, 1499(in Chinese). (冯亚栋, 张红, 程国林, 崔秀灵, 有机化学, 2014, 34, 1499.)
[9] Zhang, W.; Wang, Y.; Bai, C.; Wen, J.; Wang, N. Chin. J. Chem. 2015, 33, 401.
[10] Schmid, T. E.; Drissi-Amraoui, S.; Crévisy, C.; Baslé, O.; Mauduit, M. Beilstein J. Org. Chem. 2015, 11, 2418.
[11] Phelan, J. P.; Ellman, J. A. Beilstein J. Org. Chem. 2016, 12, 1203.
[12] Yu, Y. F.; San, F.-J.; Li, Z.-N.; Jiang, L. Chin. J. Org. Chem. 2011, 31, 443(in Chinese). (于艳飞, 单凤军, 李争宁, 姜岚, 有机化学, 2011, 31, 443.)
[13] Tang, F.-X.; Ye, J.-Y. Chin. J. Org. Chem. 2015, 35, 1414(in Chinese). (唐凤翔, 叶久勇, 有机化学, 2015, 35, 1414.)
[14] Mori, A.; Fujita, A.; Nishihara, Y.; Hiyama, T. Chem. Commun. 1997, 2159.
[15] Jordan, A. J.; Lalic, G.; Sadighi, J. P. Chem. Rev. 2016, 116, 8318.
[16] Fang, Q.; Sui, Y.-Z.; Yu, J.-L.; Xie, L.-J.; Yaag, L.-Y.; Wu, J.-W.; Wu, J. J. Hangzhou Normal Univ. (Nat. Sci. Ed.) 2015, 14, 1(in Chinese). (方嫱, 隋耀宗, 虞景露, 谢琳洁, 杨黎耀, 吴俊文, 吴静, 杭州师范大学学报(自然科学版), 2015, 14, 1.)
[17] Galestokova, Z.; Sebesta, R. Eur. J. Org. Chem. 2012, 6688.
[18] Pellissier, H. Adv. Synth. Catal. 2016, 358, 2194.
[19] Li, Z.; Liu, G.; Pauline, C. Prog. Chem. 2008, 20, 1909.
[20] Lipshutz, B. H. Synlett 2009, 509.
[21] Deutsch, C.; Krause, N.; Lipshutz, B. H. Chem. Rev. 2008, 108, 2916.
[22] Rendler, S.; Oestreich, M. Angew. Chem., Int. Ed. 2007, 46, 498.
[23] Chiu, P. Synthesis-Stuttgart 2004, 2210.
[24] Nishiyama, H.; Shiomi, T. In Metal Catalyzed Reductive C-C Bond Formation:A Departure from Preformed Organometallic Reagents, Ed.:Krische, M. J., Springer Berlin Heidelberg, Berlin, Heidelberg, 2007, p. 105.
[25] Revis, A.; Hilty, T. K. Tetrahedron Lett. 1987, 28, 4809.
[26] Chiu, P.; Leung, S. K. Chem. Commun. 2004, 2308.
[27] Lam, H. W.; Joensuu, P. M. Org. Lett. 2005, 7, 4225.
[28] Zheng, A.; Jiang, L.; Li, Z. Chin. J. Chem. 2012, 30, 2587.
[29] Lam, H. W.; Murray, G. J.; Firth, J. D. Org. Lett. 2005, 7, 5743.
[30] Lipshutz, B. H.; Amorelli, B.; Unger, J. B. J. Am. Chem. Soc. 2008, 130, 14378.
[31] Deschamp, J.; Riant, O. Org. Lett. 2009, 11, 1217.
[32] Ou, J.; Wong, W. T.; Chiu, P. Org. Biomol. Chem. 2012, 10, 5971.
[33] Zhao, D.; Oisaki, K.; Kanai, M.; Shibasaki, M. Tetrahedron Lett. 2006, 47, 1403.
[34] Li, Z.; Jiang, L.; Li, Z.; Chen, H. Chin. J. Chem. 2013, 31, 539.
[35] Zhao, D.; Oisaki, K.; Kanai, M.; Shibasaki, M. J. Am. Chem. Soc. 2006, 128, 14440.
[36] Deschamp, J.; Chuzel, O.; Hannedouche, J.; Riant, O. Angew. Chem.,Int. Ed. 2006, 45, 1292.
[37] Kato, M.; Oki, H.; Ogata, K.; Fukuzawa, S.-I. Synlett 2009, 1299.
[38] Chuzel, O.; Deschamp, J.; Chausteur, C.; Riant, O. Org. Lett. 2006, 8, 5943.
[39] Welle, A.; Diez-Gonzalez, S.; Tinant, B.; Nolan, S. P.; Riant, O. Org. Lett. 2006, 8, 6059.
[40] Li, Z.; Zhang, Z.; Yuan, L.; Jiang, L.; Li, Z.; Li, Z. Synlett 2014, 25, 724.
[41] Li, R.; Li, Z.; Jiang, L.; Li, Z. Chem. Pap. 2017, 71, 1825.
[42] Li, Z.; Li, R.; Gan, M.; Jiang, L.; Li, Z. Tetrahedron Lett. 2015, 56, 5541.
[43] Yang, T.; Zhang, Y.; Cao, P.; Wang, M.; Li, L.; Li, D.; Liao, J. Tetrahedron 2016, 72, 2707.
[44] Li, Z.; Wang, C.; Li, Z. Beilstein J. Org. Chem. 2015, 11, 213.
[45] Du, Y.; Xu, L.-W.; Shimizu, Y.; Oisaki, K.; Kanai, M.; Shibasaki, M. J. Am. Chem. Soc. 2008, 130, 16146.
[46] Suto, Y.; Kanai, M.; Shibasaki, M. J. Am. Chem. Soc. 2007, 129, 500.
[47] Li, Z.; Feng, Y.; Li, Z.; Jiang, L. Synlett 2014, 25, 2899.
[48] Oswald, C. L.; Peterson, J. A.; Lam, H. W. Org. Lett. 2009, 11, 4504.
[49] Li, Z.; Li, R.; Jiang, L.; Li, Z. Molecules 2015, 20, 15023.
[50] Miller, S. P.; Morken, J. P. Org. Lett. 2002, 4, 2743.
[51] Wong, K. C.; Ng, E.; Wong, W. T.; Chiu, P. Chem. Eur. J. 2016, 22, 3709.
[52] Linstadt, R. T. H.; Peterson, C. A.; Jette, C. I.; Boskovic, Z. V.; Lipshutz, B. H. Org. Lett. 2017, 19, 328.
[53] Reznichenko, A. L.; Nawara-Hultzsch, A. J.; Hultzsch, K. C. In Stereoselective Formation of Amines, Eds.:Li, W.; Zhang, X., Springer Berlin Heidelberg, Berlin, Heidelberg, 2014, p. 191.
[54] Huang, L.; Arndt, M.; Gooßen, K.; Heydt, H.; Gooßen, L. J. Chem. Rev. 2015, 115, 2596.
[55] Murphy, S. K.; Dong, V. M. Chem. Commun. 2014, 50, 13645.
[56] Rupnicki, L.; Saxena, A.; Lam, H. W. J. Am. Chem. Soc. 2009, 131, 10386.
[57] Soradova, Z.; Sebesta, R. ChemCatChem 2016, 8, 2581.
[58] Pirnot, M. T.; Wang, Y. M.; Buchwald, S. L. Angew. Chem., Int. Ed. 2016, 55, 48.
[59] Gribble, M. W.; Pirnot, M. T.; Bandar, J. S.; Liu, R. Y.; Buchwald, S. L. J. Am. Chem. Soc. 2017, 139, 2192.
[60] Saxena, A.; Choi, B.; Lam, H. W. J. Am. Chem. Soc. 2012, 134, 8428.
[61] Yang, Y.; Perry, I. B.; Lu, G.; Liu, P.; Buchwald, S. L. Science 2016, 353, 144.
[62] Choi, B.; Saxenaa, A.; Smith, J. J.; Churchill, G. H.; Lam, H. W. Synlett 2015, 26, 350.
[63] Ascic, E.; Buchwald, S. L. J. Am. Chem. Soc. 2015, 137, 4666.
[64] Yang, Y.; Perry, I. B.; Buchwald, S. L. J. Am. Chem. Soc. 2016, 138, 9787.
[65] Liu, R. Y.; Yang, Y.; Buchwald, S. L. Angew. Chem., Int. Ed. 2016, 55, 14077.
[66] Kokubo, K.; Miura, M.; Nomura, M. Organometallics 1995, 14, 4521.
[67] Hong, Y.-T.; Barchuk, A.; Krische, M. J. Angew. Chem., Int. Ed. 2006, 45, 6885.
[68] Fujihara, T.; Tatsumi, K.; Terao, J.; Tsuji, Y. Org. Lett. 2013, 15, 2286.
[69] Bandar, J. S.; Ascic, E.; Buchwald, S. L. J. Am. Chem. Soc. 2016, 138, 5821.
[70] Sato, K.; Isoda, M.; Ohata, S.; Morita, S.; Tarui, A.; Omote, M.; Kumadaki, I.; Ando, A. Adv. Synth. Catal. 2012, 354, 510.
[71] Miki, Y.; Hirano, K.; Satoh, T.; Miura, M. Angew. Chem., Int. Ed. 2013, 52, 10830.
[72] Miki, Y.; Hirano, K.; Satoh, T.; Miura, M. Org. Lett. 2014, 16, 1498.
[73] Zhu, S.; Niljianskul, N.; Buchwald, S. L. J. Am. Chem. Soc. 2013, 135, 15746.
[74] Zhu, S.; Buchwald, S. L. J. Am. Chem. Soc. 2014, 136, 15913.
[75] Niu, D.; Buchwald, S. L. J. Am. Chem. Soc. 2015, 137, 9716.
[76] Niljianskul, N.; Zhu, S.; Buchwald, S. L. Angew. Chem., Int. Ed. 2015, 54, 1638.
[77] Yang, Y.; Shi, S. L.; Niu, D. W.; Liu, P.; Buchwald, S. L. Science 2015, 349, 62.
[78] Xi, Y.; Butcher, T. W.; Zhang, J.; Hartwig, J. F. Angew. Chem., Int. Ed. 2016, 55, 776.
[79] Zhu, S. L.; Niljianskul, N.; Buchwald, S. L. Nat. Chem. 2016, 8, 144.
[80] Moser, R.; Boskovic, Z. V.; Crowe, C. S.; Lipshutz, B. H. J. Am. Chem. Soc. 2010, 132, 7852.
[81] Mohr, J.; Oestreich, M. Angew. Chem., Int. Ed. 2016, 55, 12148.
[82] Shi, S.-L.; Wong, Z. L.; Buchwald, S. L. Nature 2016, 532, 353.
[83] Shi, S. L.; Buchwald, S. L. Nat. Chem. 2015, 7, 38.
[84] Nishikawa, D.; Sakae, R.; Miki, Y.; Hirano, K.; Miura, M. J. Org. Chem. 2016, 81, 12128.
[85] Wang, Y.-M.; Bruno, N. C.; Placeres, A. L.; Zhu, S.; Buchwald, S. L. J. Am. Chem. Soc. 2015, 137, 10524.
[86] Semba, K.; Ariyama, K.; Zheng, H.; Kameyama, R.; Sakaki, S.; Nakao, Y. Angew. Chem., Int. Ed. 2016, 55, 6275.
[87] Friis, S. D.; Pirnot, M. T.; Buchwald, S. L. J. Am. Chem. Soc. 2016, 138, 8372.
[88] Wang, Y.-M.; Buchwald, S. L. J. Am. Chem. Soc. 2016, 138, 5024.
[89] Lee, J.; Torker, S.; Hoveyda, A. H. Angew. Chem., Int. Ed. 2017, 56, 821.
[90] Uehling, M. R.; Suess, A. M.; Lalic, G. J. Am. Chem. Soc. 2015, 137, 1424.
[91] Suess, A. M.; Uehling, M. R.; Kaminsky, W.; Lalic, G. J. Am. Chem. Soc. 2015, 137, 7747.
/
| 〈 |
|
〉 |