

碘催化亚磷酸三乙酯参与的Kabachnik-Fields反应:便捷合成含磷苯并噁嗪
收稿日期: 2017-05-16
修回日期: 2017-07-13
网络出版日期: 2017-08-16
基金资助
国家重点基础研究发展规划(973计划,No.2012CBA01204)、国家自然科学基金(No.21302084)和江西省自然科学基金(No.21302084)资助项目.
Iodine Catalyzed Kabachnik-Fields Reaction of Trialkyl Phosphites: Facile Access to Benzoxazine Containing Phosphorus
Received date: 2017-05-16
Revised date: 2017-07-13
Online published: 2017-08-16
Supported by
Project supported by the National Program on Key Basic Research Project (973 Program, No. 2012CBA01204), the National Natural Science Foundation of China (No. 21302084) and the Natural Science Foundation of Jiangxi Province (No. 20151BAB213007).
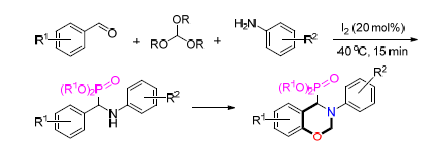
报道了由亚磷酸三乙酯通过碘催化的Kabachnik-Fields反应来合成α-氨基磷酸的方法.该方法能在40℃下快速完成,对胺、醛和亚磷酸三烷基酯具有良好的耐受性.此外,由水杨醛与亚磷酸三烷基酯水合形成的产物可在温和条件下有效地转化为含有磷酸酯的苯并噁嗪,这为新型酚醛树脂提供了新前体
关键词: 亚磷酸三烷基酯; 苯并噁嗪; Kabachnik-Fields反应; 碘
王玉凤 , 杨雅杰 , 黄玲 , 揭坤 , 郭生梅 , 蔡琥 . 碘催化亚磷酸三乙酯参与的Kabachnik-Fields反应:便捷合成含磷苯并噁嗪[J]. 有机化学, 2017 , 37(12) : 3220 -3228 . DOI: 10.6023/cjoc201705023
Iodine-catalyzed Kabachnik-Fields reaction with trialkyl phosphites for the synthesis of α-amino phosphates was developed. This transformation completes rapidly at 40℃, and is well tolerated with a range of amines and phosphites. Moreover, the products afforded by salicyl aldehydes with trialkyl phosphites could efficiently convert to benzoxazines containing phosphate under mild conditions, which provide a new precursor of new phenolic resin

Key words: trialkyl phosphite; benzoxazineis; Kabachnik-Fields reaction; iodine
[1] (a) Schmidt, R. R. Synthesis 1972, 7, 333.
(b) Burke, W. J. J. Am. Chem. Soc. 1949, 71, 609.
(c) Mueller, R.; Li, Y. X.; Hampson, A.; Zhong, S.; Harris, C.; Marrs, C.; Rachwal, R.; Ulas, J.; Nielsson, L.; Rogers, G. Bioorg. Med. Chem. Lett. 2011, 21, 3923.
(d) Dutta, A. K.; Gogoi, P.; Saikia, M.; Borah, P. R. Catal. Lett. 2016, 146, 902.
[2] (a) Bouaziz, Z.; Riondel, J.; Mey, A.; Berlion, M.; Villard, J.; Fillion, H. Eur. J. Med. Chem. 1991, 26, 469.
(b) Chylinska, J. B.; Urbanski, T.; Mordarski, M. J. Med. Chem. 1963, 6, 484.
(c) Benameur, L.; Bouaziz, Z.; Nebois, P.; Bartoli, M. H.; Boitard, M.; Fillion, H. Chem. Pharm. Bull. 1996, 44, 605.
(d) Mathew, B. P.; Kumar, A.; Sharma, S.; Shula, P. K.; Nath, M. Eur. J. Med. Chem. 2010, 45, 1502.
(e) Petrlkov, E.; Waisser, K.; DiviSova, H.; Husakov, P.; Vrabcova, P.; Kunes, J.; Kolr, K.; Stolarikov, J. Bioorg. Med. Chem. 2010, 18, 8178.
(f) Waghmode, N. A.; Kalbandhe, A. H.; Thorat, P. B.; Karade, N. N. Tetrahedron Lett. 2016, 57, 680.
[3] (a) Froimowicz, P.; Zhang, K.; Ishida, H. Chem.-Eur. J. 2016, 22, 2691.
(b) Liu, Y.-X.; Ma, H.-M.; Liu, Y.; Qiu, J.-J.; Liu, C.-M. Polymer 2016, 82, 32.
(c) Huang, C. C.; Lin, C. S.; Dai, S. A. RSC Adv. 2015, 5, 74874.
(d) Zhang, Q.; Yang, P.; Deng, Y.; Zhang, C.; Zhu, R.; Gu, Y. RSC Adv. 2015, 5, 103203.
(e) Gupta, K. S. V.; Ramana, D. V.; Vinayak, B.; Sridhar, B.; Chandrasekharam, M. New J. Chem. 2016, 40, 6389.
(f) Barta, P.; Szatmári, I.; Fülöp, F.; Heydenreich, M.; Koch, A.; Kleinpeter, E. Tetrahedron 2016, 72, 2402.
(g) Dumas, L.; Bonaud, L.; Olivier, M.; Poorteman, M.; Dubois, P. Eur. Polym. J. 2016, 75, 486.
(h) Wipt, P.; Hayes, G. B. Tetrahedron 1998, 54, 6987.
[4] (a) Su, H.; Liu, Z. J. Therm. Anal. Calorim 2013, 114, 1207.
(b) Lin, C. H.; Lin, H. T.; Sie, J. W.; Hwang, K. Y.; Tu, A. P. J. Polym. Sci.:Part A:Polym. Chem. 2010, 4555.
[5] (a) Kabachnik, M. I. Dokl. Akad. Nauk SSSR 1952, 83, 689.
(b) Fields, E. K. J. Am. Chem. Soc. 1952, 74, 1528
[6] (a) Wu, J.; Sun, W.; Wang, W.-Z.; Xia, H.-G. Chin. J. Chem. 2006, 24, 1054.
(b) Reddy, B. V. S.; Krishna, A. S.; Ganesh, A. V.; Kumar, J. J. S. N.Tetrahedron Lett. 2011, 52, 1369.
[7] Wu, J.; Sun, W.; Xia, H.-G.; Sun, X. Org. Biomol. Chem. 2006, 4, 1663.
[8] Jafari, A. A.; Nazarpour, M.; Abdollahi-Alibeik, M. Heteroat. Chem. 2010, 21, 397.
[9] Bhattacharya, T.; Majumdar, B.; Dey, D.; Sarma, T. K. RSC Adv. 2014, 4, 45831.
[10] Wu, J.; Sun, W.; Sun, X.; Xia, H.-G. Green Chem. 2006, 8, 365.
[11] (a) Ambica; Kumar, S.; Taneja, S. C.; Hundal, M. S.; Kapoor, K. K. Tetrahedron Lett. 2008, 49, 2208.
(b) Li, X.-C.; Gong, S.-S.; Zeng, D.-Y.; You, Y.-H.; Sun, Q. Tetrahedron Lett. 2016, 57, 1782.
(c) Manabe, K.; Kobayashi, S. Chem. Commun. 2000, 669.
(d) Qian, C.; Huang, T. J. Org. Chem. 1998, 63, 4125.
(e) Ranu, B. C.; Hajra, A.; Jana, U. Org. Lett. 1999, 1, 1141.
[12] (a) Tillu, V. H.; Dumbre, D. K.; Wakharkar, R. D.; Choudhary, V. R. Tetrahedron Lett. 2011, 52, 863.
(b) Kaboudin, B.; Nazari, R. Tetrahedron Lett. 2001, 42, 8211.
[13] (a) Mu, X.-J.; Lei, M.-Y.; Zou, J.-P.; Zhang, W. Tetrahedron Lett. 2006, 47, 1125.
(b) Bhattacharya, A. K.; Rana, K. C. Tetrahedron Lett. 2008, 49, 1782.
[14] (a) Ouahrouch, A.; Taourirte, M.; Schols, D.; Snoeck, R.; Andrei, G.; Angel, J. W.; Lazrek, H. B. Arch. Phram. Chem. Life Sci. 2016, 349, 30.
(b) Ouahrouch, A.; Krim, J.; Taourirte, M.; Lazrek, H. B.; Engels, J. W.; Bats, J. W. Acta Crystallogr. 2013, C69, 1157.
[15] (a) Thirumurugan, P.; Nandakumar, A.; SudhaPriya, N.; Muralidaran, D.; Perumal, P. T. Tetrahedron Lett. 2010, 51, 15708.
(b) Yadava, J. S.; Reddy, B. V. S.; Sreedhar, P. Green Chem. 2002, 4, 436.
(c) Disale, S. T.; Kale, S. R.; Kahandal, S. S.; Srinivasan, T. J.; Jayaram, R. V. Tetrahedron Lett. 2012, 53, 2277.
(d) Ordóñnez, M.; Sayago, F. J.; Cativiela, C. Tetrahedron 2012, 68, 6369.
[16] (a) Bhagat, S.; Chakraborti, A. K. J. Org. Chem. 2008, 73, 6029.
(b) Bhagat, S.; Chakraborti, A. K. J. Org. Chem. 2007, 72, 1263.
[17] Yu, Y.-Q.; Xu, D.-Z. Synthesis 2015, 47, 1869.
[18] Lee, S.; Park, J. H.; Kang, J.; Lee, J. K. Chem. Commun. 2001, 1698.
[19] Dar, B.; Singh, A.; Sahu, A.; Patida, P.; Chakraborty, A.; Sharma, M.; Singh, B. Tetrahedron Lett. 2012, 53, 5497.
[20] Kudrimoti, S.; Bommena, V. R. Tetrahedron Lett. 2005, 46, 1209
[21] (a) Huang, L.; Gong, J.; Zhu, Z.; Wang, Y.; Guo, S.; Cai, H. Org. Lett. 2017, 29, 2242.
(b) Huang, L.; Zhu, Z.; Cao, T.; Lei, X.; Gong, J.; Guo, S.; Cai, H. Chin. J. Org. Chem. 2017, 37, 1571(in Chinese).
(c) Gong, J.; Zhu, Z.; Lu, L.; Guo, S.; Cai, H. Chin. J. Org. Chem. 2015, 35, 1917(in Chinese).
(d) Gong, J.; Huang, L.; Deng, Q.; Jie, K.; Wang, Y.; Guo, S.; Cai, H. Org. Chem. Front. 2017, 4, DOI:10. 1039/C7QO00318H.
[22] Cambridge Crystallographic Data Centre (CCDC) for 4o (1509069) and 5q (1509068).
[23] (a) Sun, J.; Qiu, J.-K.; Jiang, B.; Hao, W.-J.; Guo, C.; Tu, S.-J. J. Org. Chem. 2016, 81, 3321.
(b) Ji, S.-J.; Wang, S.-Y.; Zhang Y.; Loh, T.-P. Tetrahedron 2004, 60, 2051.
(c) Zhang, H.; Wei, Q.; Zhu, G.; Qu, J.; Wang, B. Tetrahedron Lett. 2016, 57, 2633.
[24] Zhang, Y.;Zhu, C. Catal. Commun. 2011, 28, 134.
[25] Li, N.; Qiu, R.; Xu, X.; Chen, J.; Zhang, X.; Chen, S.; Yin, S. Catal. Commun. 2014, 43, 184.
[26] Thirmurugan, P.; Nandakumar, A.; Sudha, N.; Muralidaran, D.; Perumal, P. Tetrahedron. Lett. 2010, 51, 5708
[27] Song, L.; Yang, C.; Yu, Y.; Xu, D. Synthesis 2017, 49, 1641.
[28] Das, B.; Satyalakshmi, G.; Suneel, K.; Damodar, K. J. Org. Chem. 2009, 74, 8400.
[29] Shinde, p.; Kategaonkar, A.; Shingate, B.; Shingare, M. Tetrahedron Lett. 2011, 52, 2889. (Li, L.; Fan, Y.)
/
| 〈 |
|
〉 |