

茚酮并吡咯类化合物的绿色合成
收稿日期: 2017-06-01
修回日期: 2017-07-05
网络出版日期: 2017-08-18
基金资助
国家自然科学基金(Nos.21362042,21662042,U1202221,21262042),云南省自然科学基金(No.2017FA003)、云南省后备人才(No.2012HB001)、云南大学东陆学者和云南大学青年英才计划资助项目.
Green Synthesis of Indanone Fused Pyrrole Compounds
Received date: 2017-06-01
Revised date: 2017-07-05
Online published: 2017-08-18
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21362042, 21662042, U1202221, 21262042), the Natural Science Foundation of Yunnan Province (No. 2017FA003), the Talent Found in Yunnan Province (No. 2012HB001), the Donglu Scholar of Yunnan University and the Excellent Young Talents in Yunnan University.
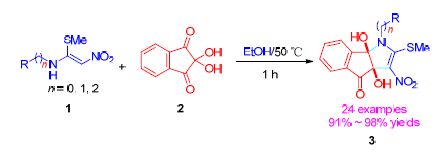
建立合成茚酮并吡咯类化合物的绿色合成方法,该方法以N-烷基-1-(甲巯基)-2-硝基乙烯胺(1)和水合茚三酮(2)为原料,在乙醇溶剂中无催化剂条件下,加热反应一步法简洁、快速地合成了一系列茚酮并吡咯类化合物.收率91%~98%.该方法具有原料易得,操作简单,路线简洁等特点.
关键词: N-烷基1-(甲巯基)-2-硝基乙烯; 茚三酮; 茚酮; 吡咯; 绿色合成
罗大云 , 胡兴梅 , 资全兴 , 林军 , 严胜骄 . 茚酮并吡咯类化合物的绿色合成[J]. 有机化学, 2017 , 37(12) : 3204 -3212 . DOI: 10.6023/cjoc201706002
green synthetic method was constructed for the synthesis of indanone fused pyrrolone compounds, which based on the catalyst-free reaction of N-alkyl-1-methylthio-2-nitroethenamine (1) with ninhydrin hydrate (2) in ethanol at reflux. As a result, a series of indanone fused pyrrolones have been synthesized with 91%~98% yields by this one-step reaction. This protocol possesses some advantages including readily available starting materials, simple operation and concise synthetic route and so on.

[1] Jones, R. A. Pyrroles,the Synthesis,Reactivity, and Physical Properties of Substituted Pyrroles, Part Ⅱ, Wiley, NewYork, 1992,
[2] Fan, H.; Peng, J. N.; Hamann, M. T.; Hu, J. F. Chem. Rev. 2008, 103, 264.
[3] Andersen, R. J.; Faulkner, D. J.; He, C. H.; Van Duyne, G. D.; Clardy, J. J. Am. Chem. Soc. 1985, 107, 5492.
[4] Fan, H.; Peng, J.; Hamann, M. T.; Hu, J. Chem. Rev. 2008, 108, 264.
[5] (a) Pla, D.; Marchal, A.; Olsen, C. A.; Francesch, A.; Cuevas, C.; Albericio, F.; Álvarez, M. J. Med. Chem. 2006, 49, 3257.
(b) Banwell, M. G.; Hamel, E.; Hockless, D. C. R.; Verdier-Pinard, P.; Willis, A. C.; Wong, D. J. Bioorg. Med. Chem. 2006, 14, 4627.
[6] Yoshida, W. Y.; Lee, K. K.; Carroll, A. R.; Scheuer, P. J. Helv. Chim. Acta 1992, 75, 1721.
[7] Kang, H.; Fenical, W. J. Org. Chem. 1997, 62, 3254.
[8] Paudler, W. W.; Kerley, G. I.; McKay, J. J. Org. Chem. 1963, 28, 2194.
[9] Powell, R. G.; Smith, C. R. Tetrahedron Lett. 1969, 46, 4081.
[10] Powell, R. G.; Weislede, D.; Smith, C. R. J. Pharm. Sci. 1972, 61, 1227.
[11] (a) Takano, I.; Yasuda, I.; Nishijima, M. Phytochemistry 1996, 44, 735.
(b) Morita, H.; Arisaka, M.; Yoshida, N. Cephalezomines A.-F. Tetrahedron 2000, 56, 2929.
(c) Morita, H.; Yoshinaga, M.; Kobayashi, J. Tetrahedron 2002, 33, 5489.
[12] (a) Wang, L.; Cherian, C.; Desmoulin, S. K.; Mitchell-Ryan, S.; Hou, Z.-J.; Matherly, L. H.; Gangjee, A. J. Med. Chem. 2012, 55, 1758.
(b) Gangjee, A.; Jain, H. D.; Queener, S. F.; Kisliuk, R. L. J. Med. Chem. 2008, 51, 4589.
(c) Deng, Y.-J.; Wang, Y.-Q.; Cherian, C.; Hou, Z.-J.; Buck, S. A.; Matherly, L. H.; Gangjee, A. J. Med. Chem. 2008, 51, 5052.
(d) Wang, L.; Desmoulin, S.; Cherian, K. C.; Polin, L.; White, K.; Kushner, J.; Fulterer, A.; Chang, M.-H.; Mitchell-Ryan, S. M.; Romero, M. F.; Hou, Z.-J.; Matherly, L. H.; Gangjee, A. J. Med. Chem. 2011, 54, 7150.
[13] Regueiro-Ren, A.; Xue, Q.-M.; Swidorski, J. J.; Gong, Y.-F.; Mathew, M.; Parker, D. D.; Yang, Z.; Eggers, B.; D'Arienzo, C.; Sun, Y.-N.; Malinowski, J.; Gao, Q.; Wu, D.-D.; Langley, D. R.; Colonno, R. J.; Chien, C.; Grasela, D. M.; Zheng, M.; Lin, P.-F.; Meanwell, N. A.; Kadow, J. F. J. Med. Chem. 2013, 56, 1656.
[14] Varaprasad, C. V. N. S.; Ramasamy, K. S.; Girardet, J.-L.; Gunic, E.; Lai, V.; Zhong, Z.; An, H.; Hong, Z. Bioorg. Chem. 2007, 35, 25.
[15] (a) Pudio, J. S.; Saxena, N. K.; Nassiri, M. R.; Turk, S. R.; Drach, J. C.; Townsend, L. B. J. Med. Chem. 1988, 31, 2086.
(b) Saxena, N. K.; Hagenow, B. M.; Genzlinger, G.; Turk, S. R.; Drach, J. C.; Townsend, L. B. J. Med. Chem. 1988, 31, 1501.
[16] E1-Gaby, M. S. A. A.; Gaber, M.; Atalla, A. A.; Abd Al-Wahab, K. A. Farmaco 2002, 57, 613.
[17] Trzoss, M.; Bensen, D. C.; Lia, X.-M.; Chen, Z.-Y.; Lam, T.; Zhang, J.-Z.; Creighton, C. J.; Cunningham, M. L.; Kwan, B.; Stidham, M.; Nelson, K.; Brown-Driver, V.; Castellano, A.; Shaw, K. J.; Lightstone, F. C.; Wong, S.-E.; Nguyen, T. B.; Finn, J.; Tari, L. W. Bioorg. Med. Chem. Lett. 2013, 23, 1537.
[18] (a) Estévez, V.; Villacampa, M.; Menéndez, J. C. Chem. Soc. Rev. 2010, 39, 4402.
(b) Preller, M.; Chinthalapudi, K.; Martin, R.; Knölker, H.-J.; Manstrin, D. J. J. Med. Chem. 2011, 54, 3675.
[19] Chen, Z.-C.; Venkatesan, A. M.; Dehnhardt, C. M.; Ayral-Kaloustian, S.; Brooijmans, N.; Mallon, R.; Feldberg, L.; Hollander, I.; Lucas, J.; Yu, K.; Kong, F.-M.; Mansour, T. S. J. Med. Chem. 2010, 53, 3169.
[20] Weinberg, L. R.; Albom, M. S.; Angeles, T. S.; Breslin, H. J.; Gingrich, D. E.; Huang, Z.-Q.; Lisko, J. G.; Mason, J. L.; Milkiewicz, K. L.; Thieu, T. V.; Underiner, T. L.; Wells, G. J.; Wells-Knecht, K. J.; Dorsey, B. D. Bioorg. Med. Chem. Lett. 2011, 21, 7325.
[21] Lindsay, K. B.; Pyne, S. G. Tetrahedron 2004, 60, 4173.
[22] (a) Liu, Y.; Fang, J.-P.; Cai, H.-Y.; Xiao, F.; Ding, K.; Hua, Y.-H.; Bioorg. Med. Chem. 2012, 20, 5473.
(b) Palmer, A. M.; Münch, G.; Brehm, C.; Zimmermann, P. J.; Buhr, W.; Feth, M. P.; Simon, W. A. Bioorg. Med. Chem. 2008, 16, 1511.
[23] (a) Fan, Y.; Liu, S.; Chen, N.; Shao, X.; Xu, X.; Li, Z. Synlett 2015, 26, 393.
(b) Chen, X.-B.; Wang, X.-Y.; Zhu, D.-D.; Yan, S.-J.; Lin, J. Tetrahedron 2014, 70, 1047.
(c) Chen, X.-B.; Yan, S.-J.; Su, A.; Liu, W.; Lin, J. Tetrahedron 2015, 71, 4745.
(d) Hu, L.; Wang, K.-M.; Zhao, M.; Lin, X.-R.; Zju, H.-Y.; Yan, S.-J.; Lin, J. Tetrahedron 2014, 70, 4478.
(e) Chen, X.-B.; Liu, Z.-C.; Yang, L.-F.; Yan, S.-J.; Lin, J. ACS Sustainable Chem. Eng. 2014, 2, 1155.
(f) Chen, X.-B.; Liu, Z.-C.; Lin, X.-R.; Huang, R.; Yan, S.-J.; Lin, J. ACS Sustainable Chem. Eng. 2014, 2, 2391.
(g) Wang, K.-M.; Yan, S.-J.; Lin, J. Eur. J. Org. Chem. 2014, 1129.
[24] (a) Li, X.-L.; Wang, J.-Q.; Li, L.; Yin, Y.-W.; Ye, L.-W. Acta Chim. Sinica 2016, 74, 49(in Chinese). (李新玲, 王佳琪, 李龙, 尹应武, 叶龙武, 化学学报, 2016, 74, 49.)
(b) Shen, B.-X.; Qian, Y. Chin. J. Org. Chem. 2016, 36, 774(in Chinese). (沈宝星, 钱鹰, 有机化学, 2016, 36, 774.)
(c) Chen, J.; Zhang, Y.-K.; Zhan, X.-P.; Liu, Z.-L.; Mao, Z.-M. Chin. J. Org. Chem. 2016, 36, 572(in Chinese). (陈简, 张袁魁, 詹晓平, 刘增路, 毛振民, 有机化学, 2016, 36, 572.)
(d) Li, Z.; Yan, N.; Xie, J.; Liu, P.; Zhang, J.; Dai, B. Chin. J. Chem. 2015, 33, 589.
[25] (a) Zhou, B.; Liu, Z.-C.; Qu, W.-W.; Yang, R.; Lin, X.-R.; Yan, S.-J.; Lin, J. Green Chem. 2014, 16, 4359.
(b) Zeng, C.-C.; Liu, F.-J.; Ping, D.-W.; Hu, L.-M.; Cai, Y.-L.; Zhong, R.-G. J. Org. Chem. 2009, 74, 6386.
(c) Liu, J.; Zhang, H.-R.; Lin, X.-R.; Yan, S.-J.; Lin, J. RSC Adv. 2014, 4, 27582.
(d) Wang, K.-M.; Ma, Y.-L.; Lin, X.-R.; Yan, S.-J.; Lin, J. RSC Adv. 2015, 5, 36472.
(e) Chen, X.-B.; Zhu, L.; Fang, L.; Yang, S.-J.; Lin, J. RSC Adv. 2014, 4, 9926.
(f) Yu, F.-C.; Hao, X.-P.; Lin, X.-R.; Yan, S.-J.; Lin, J. Tetrahedron 2015, 71, 4084.
(g) Kong, L.; Yang, R.; Du, X.; Yan, S.; Lin, J. Chin. J. Org. Chem. 2016, 36, 2437(in Chinese). (孔令斌, 杨瑞霞, 杜璇璇, 严胜骄, 林军, 有机化学, 2016, 36, 2437.)
[26] (a) Saito, A.; Umakoshi, M.; Yagyu, N.; Hanzawa, Y. Org. Lett. 2008, 10, 1783.
(b) Duarte, C. D.; Barreiro, E. J.; Fraga, C. A. Mini-Rev. Med. Chem. 2007, 7, 1108.
(c) Huang, L.; Lu, C.; Sun, Y.; Mao, F.; Luo, Z.; Su, T.; Jiang, H.; Shan, W.; Li, X. J. Med. Chem. 2012, 55, 8483.
(d) Guillon, J.; Dallemagne, P.; Leger, J.-M.; Sopkova, J.; Bovy, P. R.; Jarry, C.; Rault, S. Bioorg. Med. Chem. 2002, 10, 1043.
(e) Yu, F.-C.; Yan, S.-J.; Hu, L.; Wang, Y.-C.; Lin, J. Org. Lett. 2011, 13, 4782.
(f) Xu, H.; Zhou, B.; Zhou, P.; Zhou, J.; Shen, Y.; Yu, F.-C.; Lu, L.-L. Chem. Commun. 2016, 52, 8002.
(g) Xu, H.; Zhou, P.; Zhou, B.; Zhou, J.; Shen, Lu, L.-L. RSC Adv. 2016, 6, 73760.
[27] Patel, A.; Giles, D.; Basavarajaswamy, G.; Sreedhar, C.; Patel, A. Med. Chem. Res. 2012, 21, 4403.
[28] Mohil, R.; Kumar, D.; Mor, S. J. Heterocycl. Chem. 2014, 51, 203.
[29] (a) Insuasty, B.; Orozco, F.; Lizarazo, C.; Quiroga, J. Bioorg. Med. Chem. 2008, 16, 8492.
(b) Saxena, H. O.; Faridi, U.; Srivastava, S.; Kumar, J. K.; Darokar, M. P.; Luqman, S.; Chanotiya, C. S.; Krishna, V.; Negi, A. S.; Khanuja, S. P. S. Bioorg. Med. Chem. Lett. 2008, 18, 3914.
(c) Lobo, G.; Monasterios, M.; Rodrigues, J.; Gamboa, N.; Capparelli, M. V.; Martínezcuevas, J.; Lein, M.; Jung, K.; Abramjuk, C.; Charris, J. Eur. J. Med. Chem. 2015, 96, 281.
[30] Finkielsztein, L. M.; Castro, E. F.; Fabiaan, L. E.; Moltrasio, G. Y.; Campos, R. H.; Cavallaro, L. V.; Moglioni, A. G. Eur. J. Med. Chem. 2008, 43, 1767.
[31] Chen, N.; Meng, X.; Zhu, F.; Cheng, J.; Shao, X.; Li, Z. J. Agric. Food Chem. 2015, 63, 1360.
[32] Xichuan, M.-M.; Chi, T.-X.; Nan, J.-Z. CN 93104329, 1993[Chem. Abstr. 1994, 120, 244386].
[33] Jabor, G. G.; Bouaziz, Z.; Winter, E.; Daflonyunes, N.; Aichele, D.; Nacereddine, A.; Marminon, C.; Valdameri, G.; Zeinyeh, W.; Bollacke, A.; Guillon, J.; Lacoudre, A.; Pinaud, N.; Cadena, S. M.; Jose, J.; Borgne, M. L.; Pietro, A. D. J. Med. Chem. 2015, 58, 265.
[34] Nugiel, D. A.; Vidwans, A.; Etzkorn, A. M.; Rossi, K. A.; Benfield, P. A.; Burton, C. R.; Cox, S.; Doleniak, D.; Seitz, S. P. J. Med. Chem. 2002, 45, 5224.
[35] Usui, T.; Ban, H. S.; Kawada, J.; Hirokawa, T.; Nakamura, H. Bioorg. Med. Chem. Lett. 2008, 18, 285.
[36] (a) Dhareshwar, S. S.; Stella, V. J. J. Pharm. Sci. 2010, 99, 2711.
(b) Usta, H.; Facchetti, A.; Marks, J. T. Org. Lett. 2008, 10, 1385.
(c) Shultz, D. A.; Sloop, J. C.; Washington, G. J. Org Chem. 2006, 71, 9104.
[37] (a) Suchand, B.; Satyanarayana, G. J. Org. Chem. 2017, 82, 372.
(b) Zhao, P.; Liu, Y.; Xi, C. Org. Lett. 2015, 17, 4388.
(c) Domaradzki, M. E.; Long, Y.; She, Z.; Liu, X.; Zhang, G.; Chen, Y. J. Org. Chem. 2015, 80, 11360.
(d) Kashanna, J.; Nagaraju, K.; Kumara Swamy, K. C. Tetrahedron Lett. 2016, 57, 1576.
(e) Zhao, P.; Liu, Y.; Xi, C. Org. Lett. 2015, 17, 4388.
(f) Pan, C.; Huang, B.; Hu, W.; Feng, X.; Yu, J.-T. J. Org. Chem. 2016, 81, 2087.
[38] (a) Estévez, V.; Villacampa, M.; Menéndez, J. C. Chem. Soc. Rev. 2014, 43, 4633.
(b) Estévez, V.; Villacampa, M.; Menéndez, J. C. Chem. Soc. Rev. 2010, 39, 4402.
(c) Vessally, E. RSC Adv. 2016, 6, 18619.
(d) Torres, G. M.; Quesnel, J. S.; Bijou, D.; Arndtsen, B. A. J. Am. Chem. Soc. 2016, 138, 7315.
(e) Chen, N.; Zou, M.; Tian, X.; Zhu, F.; Jiang, D.; Cheng, J. Eur. J. Org. Chem. 2014, 2014, 6210.
(f) Liu, X. M.; Lin, X. R.; Yan, S. J.; Peng, M. Y.; Huang, R.; Lin, J. Tetrahedron 2016, 72, 5314.
[39] (a) Schertz, T. D; Reiter, R. C.; Stevenson, C. D. J. Org. Chem. 2001, 66, 7596.
(b) Soro, Y.; Bamba, F.; Siaka, S.; Coustard, J. M. ChemInform 2016, 37, 3315.
/
| 〈 |
|
〉 |