

半三明治型铱催化剂催化氢化葡萄糖制备山梨糖醇
收稿日期: 2017-08-01
修回日期: 2017-08-31
网络出版日期: 2017-09-15
基金资助
国家自然科学基金(Nos.21572212,21402181,21325208)、中国科学院项目基金(Nos.XDB20000000,YZ201563)、中央高校基础研究经费、教育部长江学者和创新团队发展计划、安徽省科技攻关计划(No.1604a0702027)资助项目.
Preparation of D-Sorbitol by Catalytic Hydrogenation of D-Glucose with Semi Sandwich Iridium Catalyst
Received date: 2017-08-01
Revised date: 2017-08-31
Online published: 2017-09-15
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21572212, 21402181, 21325208), the Science Foundation of the Chinese Academy of Sciences (Nos. XDB20000000, YZ201563), the Fundamental Research Funds for the Central Universities, the Program for Changjiang Scholars and Innovative Research Team in University of the Ministry of Education of China, the Key Technologies R&D Programme of Anhui Province (No. 1604a0702027).
龚宝祥 , 严龙 , 陈蒙远 , 邓晋 , 傅尧 . 半三明治型铱催化剂催化氢化葡萄糖制备山梨糖醇[J]. 有机化学, 2017 , 37(12) : 3170 -3176 . DOI: 10.6023/cjoc201708001
[Cp*Ir-(di-OH-bpy)(OH2)] [SO4] (di-OH-bpy=4,4'-dihydroxyl-2,2'-bipyridine) exhibited high catalytic performance during the hydrogenation of D-glucose to D-sorbitol. Using formic acid as hydrogen source led to an unsatisfactory D-sorbitol selectivity. The ring-opening hydrogenation of glucose was efficiently achieved by hydrogen. Under optimal conditions, D-sorbitol could be obtained in 96% yield. The effect of different ligands in iridium catalyst on hydrogenation was also investigated. A speculated mechanism was proposed to illustrate the detailed process of D-glucose hydrogenation to produce sorbitol. The catalytic system has the merits of relatively mild reaction conditions and high product selectivity, which provides an effective method for the catalytic hydrogenation of other biomass-based platform chemicals.
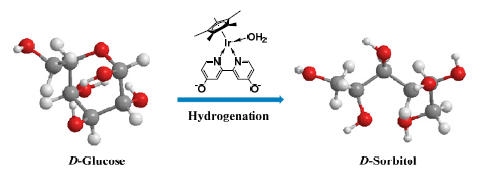
Key words: glucose; sorbitol; Cp*Ir; homogeneous hydrogenation; bipyridine ligand
[1] Corma, A.; Iborra, S.; Velty, A. Chem. Rev. 2007, 107, 2411.
[2] (a) Delidovich, I.; Hausoul, P. J. C.; Deng, L.; Pfuzenreuter, R.; Rose, M.; Palkovits, R. Chem. Rev. 2016, 116, 1540.
(b) Yang, Z.; Fu, Y.; Guo, Q. X. Chin. J. Org. Chem. 2014, 34, 273(in Chinese). (杨珍, 傅尧, 郭庆祥, 有机化学, 2014, 34, 273.)
[3] (a) Wang, H. J.; Zhao, Y.; Wang, C.; Fu, Y.; Guo, Q. X. Acta Chim. Sinica 2009, 67, 893(in Chinese). (王华静, 赵岩, 王晨, 傅尧, 郭庆祥, 化学学报, 2009, 67, 893.)
(b) Regalbuto, J. R. Science 2009, 325, 822.
(c) Holm, M. S.; Saravanamurugan, S.; Taarning, E. Science 2010, 328, 602.
(d) Luo, C.; Wang, S.; Liu, H. Angew. Chem., Int. Ed. 2007, 46, 7636.
(e) Ragauskas, A. J.; Williams, C. K.; Davison, B. H.; Britovsek, G.; Cairney, J.; Eckert, C. A.; Hallett, J. P.; Leak, D. J.; Liotta, C. L.; Mielenz, J. R.; Murhy, R.; Templer, R.; Tschaplinski. T. Science 2006, 311, 484.
(f) Li, J.; Huang, Y. B.; Guo, Q. X.; Fu, Y. Acta Chim. Sinica 2014, 72, 1223(in Chinese). (李江, 黄耀兵, 郭庆祥, 傅尧, 化学学报, 2014, 72, 1223.)
[4] van Putten, R. J.; van der Waal, J. C.; de Jong, E.; Rasrendra, C. B.; Heeres, H. J.; de Vries, J. G. Chem. Rev. 2013, 113, 1499.
[5] Climent, M. J.; Corma, A.; Iborra, S. Green Chem. 2011, 13, 520.
[6] Zhang, J.; Li, J. B.; Wu, S. B.; Liu, Y. Ind. Eng. Chem. Res. 2013, 52, 11799.
[7] Vilcocq, L.; Cabiac, A.; Especel, C.; Guillon, E.; Duprez, D. Oil Gas Sci. Technol. 2013, 68, 841.
[8] Wang, Y.; De, S.; Yan, N. Chem. Commun. 2016, 52, 6210.
[9] Brahme, P. H.; Doraiswamy, L. K. Ind. Eng. Chem. Process Des. Dev. 1976, 15, 130
[10] Wisnlak, J.; Simon, R. Ind. Eng. Chem. Prod. Res. Dev. 1979, 18, 50.
[11] Gallezot, P.; Cerino, P. J.; Blanc, B.; Fleche, G.; Fuertes. P. J. Catal. 1994, 146, 93.
[12] Kusserow, B.; Schimpf, S.; Claus, P. Adv. Synth. Catal. 2003, 345, 289.
[13] Qiao, M. H.; Xie, S. H.; Dai, W. L.; Deng, J. F. Acta Chim. Sinica 2000, 58, 904(in Chinese). (乔明华, 谢颂海, 戴维林, 邓景发, 化学学报, 2000, 58, 904.)
[14] Wisnlak, J.; Simon, R. Ind. Eng. Chem. Prod. Res. Dev. 1979, 18, 50.
[15] Lazaridis, P. A.; Karakoulia, S.; Delimitis, A.; Coman, S. M.; Parvulescu, V. I.; Triantafyllidis, K. S. Catal. Today. 2015, 257, 281.
[16] Perrard, A.; Gallezot, P.; Joly, J. P.; Durand, R.; Baljou, C.; Coq, B.; Trens, P. Appl. Catal. A 2007, 331, 100.
[17] Tathod, A.; Kane, T.; Sanil, E. S.; Dhepe, P. L. J. Mol. Catal. A:Chem. 2014, 388, 90.
[18] Zhang, X.; Durndell, L. J.; Isaacs, M. A.; Parlett, C. M.; Lee, A. F.; Wilson, K. ACS Catal. 2016, 6, 7409.
[19] Liu, J.; Bai, P.; Zhao, X. S. Phys. Chem. Chem. Phys. 2011, 13, 3758.
[20] Zhang, J.; Lin, L.; Zhang, J.; Shi, J. Carbohydr. Res. 2011, 346, 1327.
[21] Tukacs, J. M.; Király, D.; Strádi, A.; Novodarszki, G.; Eke, Z.; Dibó, G.; Mika, L. T. Green Chem. 2012, 14, 2057.
[22] Qi, L.; Horvaath, I. T. ACS Catal. 2012, 2, 2247.
[23] Kolaric, S.; Šunjic, V. J. Mol. Catal. A:Chem. 1996, 110, 189.
[24] Tan, X. F.; Wang, G. Z.; Zhu, Z. Y.; Ren, C. H.; Zhou, J. P.; Lv, H.; Zhang, X. Y.; Chung, L. W.; Zhang, L.; Zhang, X. M. Org. Lett. 2016, 18, 1518.
[25] Himeda, Y.; Onozawa-Komatsuzaki, N.; Miyazawa, S.; Sugihara, H.; Hirose, T.; Kasuga, K. Chem.-Eur. J. 2008, 14, 11076.
[26] Wang, W. H.; Hull, J. F.; Muckerman, J. T.; Fujita, E.; Himeda, Y. Energy Environ. Sci. 2012, 5, 7923.
[27] Hull, J. F.; Himeda, Y.; Wang, W. H.; Hashiguchi, B.; Periana, R.; Szalda, D. J.; Fujita, E. Nat. Chem. 2012, 4, 383.
[28] Himeda, Y. Eur. J. Inorg. Chem. 2007, 25, 3927.
[29] Himeda, Y.; Onozawa-Komatsuzaki, N.; Sugihara, H.; Kasuga, K. Organometallics 2007, 26, 702.
[30] Himeda, Y.; Onozawa-Komatsuzaki, N.; Sugihara, H.; Kasuga, K. J. Photochem., Photobiol., A 2006, 182, 306.
[31] Himeda, Y.; Onozawa-Komatsuzaki, N.; Sugihara, H.; Kasuga, K. J. Am. Chem. Soc. 2005, 127, 13118.
[32] Ogo, S.; Kabe, R.; Hayashi, H.; Harada, R.; Fukuzumi, S. Dalton Trans. 2006, 39, 4657.
[33] Deng, J.; Wang, Y.; Pan, T.; Xu, Q.; Guo, Q. X.; Fu, Y. ChemSusChem 2013, 6, 1163.
[34] (a) Wu, W. P.; Xu, Y. J.; Zhu, R.; Cui, M. S.; Li, X. L.; Deng, J.; Fu, Y. ChemSusChem 2017, 9, 1209.
(b) Wu, W. P.; Xu, Y. J.; Chang, S. W.; Deng, J.; Fu, Y. ChemCatChem 2016, 8, 3375.
(c) Xu, Y. J.; Shi, J.; Wu, W. P.; Zhu, R.; Li, X. L.; Deng, J.; Fu, Y. Appl. Catal. A:Gen. 2017, 543, 266.
[35] Fujita, E.; Muckerman, J. T.; Himeda, Y. Biochim. Biophys. Acta,Bioenerg. 2013, 1827, 1031.
[36] Wang, W. H.; Himeda, Y.; Muckerman, J. T.; Manbeck, G. F.; Fujita, E. Chem. Rev. 2015, 115, 12936.
[37] Xu, Z. W.; Yan, P. F.; Zhang, Z. Chin. J. Org. Chem. 2017, 37, 40(in Chinese). (许占威, 颜佩芳, 张宗超, 有机化学, 2017, 37, 40.)
[38] Weiss, J. Trans. Faraday Soc. 1941, 37, 782.
[39] Brewster, T. P.; Miller, A. J.; Heinekey, D. M.; Goldberg, K. I. J. Am. Chem. Soc. 2013, 135, 16022.
/
| 〈 |
|
〉 |