

吡啶乙醇类双[N,O]环钯配合物在Fujiwara-Moritani反应中的高效催化应用
收稿日期: 2017-08-30
修回日期: 2017-09-19
网络出版日期: 2017-09-26
基金资助
国家自然科学基金(Nos.21172200,21302172)资助项目.
Highly Catalytic Activity of Bis(alkoxo)palladium Complexes for Fujiwara-Moritani Reaction
Received date: 2017-08-30
Revised date: 2017-09-19
Online published: 2017-09-26
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21172200, 21302172).
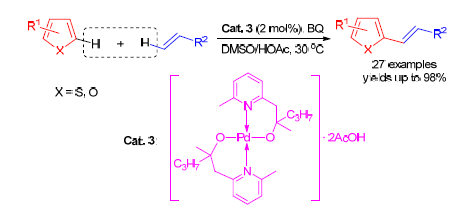
在前期工作的基础上,设计合成了一系列吡啶乙醇类双[N,O]环钯配合物,以该类环钯配合物为催化剂,通过Fujiwara-Moritani反应,在相对温和的反应条件下(2 mol%催化剂,30℃,空气环境中)即可高效实现噻吩、呋喃类芳杂环与各类烯烃的偶联,并以中等到高等的分离产率得到相应的目标化合物.进一步通过动力学实验和ESI(+)-MS同步检测,推测该反应是通过协同的金属化/去质子化(CMD)机理进行.
李亚波 , 申振 , 黄萌萌 , 张建业 , Jung Keun Kim , 吴养洁 . 吡啶乙醇类双[N,O]环钯配合物在Fujiwara-Moritani反应中的高效催化应用[J]. 有机化学, 2018 , 38(1) : 200 -207 . DOI: 10.6023/cjoc201708063
A series of bis(alkoxo)palladium complexes (2 mol%) based on pyridine-containing alcohol ligand were tested for Fujiwara-Moritani reaction of thiophenes/furans with various olefins. The desired products were isolated in moderate to excellent yields under mild conditions. A possible concerted metalation-deprotonation (CMD) pathway for this transformation was proved by control experiments and ESI(+)-MS analysis.

[1] (a) Chen, X.; Engle, K. M.; Wang, D.-H.; Yu, J.-Q. Angew. Chem., Int. Ed. 2009, 48, 5094.
(b) Lyons, T. W.; Sanford, M. S. Chem. Rev. 2010, 110, 1147.
(c) Le Bras, J.; Muzart, J. Chem. Rev. 2011, 111, 1170.
(d) Shang, X.-J.; Liu, Z.-Q. Chem. Soc. Rev. 2013, 42, 3253.
(e) Shang, X.-J.; Liu, Z.-Q. Chin. J. Org. Chem. 2015, 35, 522(in Chinese).(尚筱洁, 刘忠全, 有机化学, 2015, 35, 522.)
[2] (a) Asano, R.; Moritani, I.; Fujiwara, Y.; Teranishi, S. Bull. Chem. Soc. Jpn. 1973, 46, 663.
(b) Fujiwara, Y.; Maruyama, O.; Yoshidomi, M.; Taniguchi, H. J. Org. Chem. 1981, 46, 851.
(c) Jia, C.-G.; Lu, W.-J.; Kitamura, T.; Fujiwara, Y. Org. Lett. 1999, 1, 2097.
[3] (a) Maehara, A.; Satoh, T.; Miura, M. Tetrahedron 2008, 64, 5982.
(b) Takeda, D.; Hirano, K.; Satoh, T.; Miura, M. Org. Lett. 2013, 15, 1242.
(c) Iitsuka, T.; Schaal, P.; Hirano, K.; Satoh, T.; Bolm, C.; Miura, M. J. Org. Chem. 2013, 78, 7216.
[4] (a) Aouf, C.; Thiery, E.; Le Bras, J.; Muzart, J. Org. Lett. 2009, 11, 4096.
(b) Vasseur, A.; Muzart, J.; Le Bras, J. Chem. Eur. J. 2011, 17, 12556.
(c) Vasseur, A.; Harakat, D.; Muzart, J.; Le Bras, J. J. Org. Chem. 2012, 77, 5751.
(d) Vasseur, A.; Harakat, D.; Muzart, J.; Le Bras, J. Adv. Synth. Catal. 2013, 355, 59.
[5] (a) Itahara, T.; Ouseto, F. Synthesis 1984, 488.
(b) Zhao, J.-L.; Huang, L.-H.; Cheng, K.; Zhang, Y.-H. Tetrahedron Lett. 2009, 50, 2758.
(c) Zhang, Y.; Li, Z.; Liu, Z.-Q. Org. Lett. 2012, 14, 226.
(d) Wang, F.; Hu, Y.-C.; Shen, A.; Cao, Y.-C. Chin. J. Org. Chem. 2017, 37, 2050(in Chinese).(王凡, 胡宇才, 沈安, 曹育才, 有机化学, 2017, 37, 2050.)
[6] Li, Y.-B.; Mi, X.; Huang, M.-M.; Cai, R.-R.; Wu, Y.-J. Tetrahedron 2012, 68, 8502.
[7] (a) Li, Y.-B.; Wang, J.-R.; Huang, M.-M.; Wang, Z.-W.; Wu, Y.-S.; Wu, Y.-J. J. Org. Chem. 2014, 79, 2890.
(b) Li, Y.-B.; Wang, J.-R.; Yan, B.-Q.; Huang, M.-M.; Zhu, Y.; Wu, Y.-S.; Wu, Y.-J. Tetrahedron 2015, 71, 2729.
[8] Wang, Z.-W.; Li, Y.-B.; Yan, B.-Q.; Huang, M.-M.; Wu, Y.-J. Synlett 2015, 26, 531.
[9] Gorelsky, S. I.; Lapointe, D.; Fagnou, K. J. Org. Chem. 2012, 77, 658.
[10] Nam, Ng. H.; Buu-Hoi, Ng. Ph.; Xuong, Ng. D. J. Chem. Soc. 1954, 1690.
[11] Kantchev, E. A. B.; Peh, G.-R.; Zhang, C.; Ying, J. Y. Org. Lett. 2008, 10, 3949.
[12] Wang, Z.; Pitteloud, J.-P.; Montes, L.; Rapp, M.; Derane, D.; Wnuk, S. F. Tetrahedron 2008, 64, 5322.
[13] El-Batta, A.; Jiang, C.; Zhao, W.; Anness, R.; Cooksy, A. L.; Bergdahl, M. J. Org. Chem. 2007, 72, 5244.
[14] Francesco B.; Giorgio L.; Bruno, M. Gazz. Chim. Ital. 1969, 99, 762.
[15] Berthelot, P.; Vaccher, C.; Flouquet, N.; Debaert, M.; Luyckx, M.; Brunet, C. J. Med. Chem. 1991, 34, 2557.
/
| 〈 |
|
〉 |