

金属钼酸类催化剂在双氧水作氧化剂下选择性氧化烯烃和醇
Metal Molybdate Catalysts for the Selective Oxidation of Olefins and Alcohols Using Hydrogen Peroxide as Oxidant
Received date: 2017-07-12
Revised date: 2017-08-24
Online published: 2017-10-11
胡传峰 , 周建豪 , 黄志达 , 傅惠惠 , 彭新华 . 金属钼酸类催化剂在双氧水作氧化剂下选择性氧化烯烃和醇[J]. 有机化学, 2018 , 38(2) : 486 -491 . DOI: 10.6023/cjoc201707017
Several facile metal molybdates were prepared by hydrothermal method and characterized by X-ray diffraction (XRD). Meanwhile, the bimetallic combination effect of these metal molybdates as bimetallic catalysts applied to the selective oxidation of various olefins and alcohols was investigated. It was found that there was the existence of bimetallic combination effect and the combination of Co and Mo had benignant effect compared with the individual metal catalysts and other metal molybdates in the reaction of oxidizing olefins to aldehydes. This bimetallic catalyst also showed very prominent performance (selectivity is 100%) in the oxidation of the alcohols to corresponding carbonyl compounds. The combination of double metal provides a method for the catalytic oxidation of olefins and alcohols.
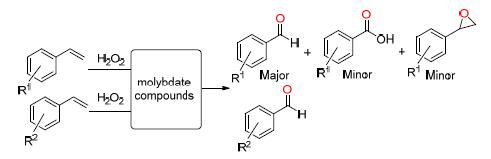
[1] Pathan, S.; Patel, A. Ind. Eng. Chem. Res. 2013, 52, 11913.
[2] Mi, C.; Meng, X. G.; Liao. X. H.; Peng, X. RSC Adv. 2015, 5, 69487.
[3] Shi, F.; Tse, M. K.; Pohl, M. M.; Radnik, J.; Brückner, A.; Zhang, S.; Beller, M. J. Mol. Catal. A:Chem. 2008, 292, 28.
[4] (a) Amini, M.; Naslhajian, H.; Farnia, S. M. F. New J. Chem. 2014, 38, 1581.
(b) Layek, K.; Maheswaran, H.; Arundhathi, R.; Kantam, M. L.; Bhargava, S. K. Adv. Synth. Catal. 2011, 353, 606.
(c) Uyanik, M.; Fukatsu, R.; Ishihara, K. Chem. Asian J. 2010, 5, 456.
[5] (a) Zhan, W. C.; Guo, Y. L.; Wang, Y. Q.; Liu, X. H.; Guo, Y.; Wang, Y. S.; Zhang, Z. G.; Lu, G. Z. J. Phys. Chem. B 2007, 111, 12103.
(b) Gooßen, L. J.; Khan, B. A.; Fett, T.; Treu, M. Adv. Synth. Catal. 2010, 352, 2166.
(c) Shirini, F.; Zolfigol, M. A.; Shahriari, A. J. Iran. Chem. Soc. 2008, 5, 420.
[6] (a) Yadav, G. D.; Mistry, C. K. J. Mol. Catal. A:Chem. 1995, 102, 67.
(b) Lee, K.; Maleczka, R. E. J. Org. Chem. 1999, 64, 342.
[7] (a) Yadav, G. D.; Haldavanekar, B. V. J. Phys. Chem. A 1997, 101, 36.
(b) Dibble, D. J.; Ziller, J. W.; Woerpel, K. A. J. Org. Chem. 2011, 76, 7706.
[8] Rao, K. T. V.; Rao, P. S. N.; Nagaraju, P.; Prasad, P. S. S.; Lingaiah, N. J. Mol. Catal. A:Chem. 2009, 303, 84.
[9] Bentrup, U.; Brückner, A.; Martin, A.; Lücke, B. J. Mol. Catal. A:Chem. 2000, 162, 391.
[10] Xia, H.; Liu, Z. L.; Xu, Y. Y.; Zuo, J. L.; Qin, Z. Z. Catal. Commun. 2016, 86, 72.
[11] Criegee, R. Angew. Chem. 1975, 14, 745.
[12] (a) Yu, W. S.; Mei, Y.; Kang, Y.; Hua, Z. M.; Jin, Z. D. Org. Lett. 2004, 6, 3217.
(b) Cui, N.; Zhao, Y.; Wang Y. X. Chin. J. Org. Chem. 2017, 37, 20(in Chinese). (崔娜, 赵宇, 王云侠, 有机化学, 2017, 37, 20.)
[13] Das, A.; Ghosh, T. K.; Chowdhury, A. D.; Mobin, S. M.; Lahiri, G. K. Polyhedron 2013, 52, 1130.
[14] Li, N.; Gao, Y.; Zhang, X.; Yu, Z.; Shi, L.; Sun, Q. Chin. J. Catal. 2015, 36, 721.
[15] Thao, N. T.; Huyen, L. T. K. Chem. Eng. J. 2015, 279, 840.
[16] Yang, F.; Zhou, S.; Gao, S.; Liu, X.; Long, S.; Kong, Y. Microporous Mesoporous Mater. 2017, 238, 69.
[17] Nyawade, E. A.; Friedrich, H. B.; Omondi, B.; Mpungose, P. Organometallics 2015, 34, 4922.
[18] Zhou, W.; Liu, J.; Pan, J.; Sun, F.; He, M.; Chen, Q. Catal. Commun. 2015, 69, 1.
[19] Thao, N. T.; Trung, N. D.; Long, D. V. Catal. Lett. 2016, 146, 918.
[20] Jafarpour, M.; Ghahramaninezhad, M.; Rezaeifard, A. RSC Adv. 2014, 4, 1601.
[21] Pardeshi, S. K.; Pawar, R. Y. J. Mol. Catal. A:Chem. 2011, 334, 35.
[22] Pathan, S.; Patel, A. Dalton Trans. 2011, 40, 348.
[23] Pathan, S.; Patel, A. Chem. Eng. J. 2014, 243, 183.
[24] (a) Maurya, M. R.; Kumar, N. J. Mol. Catal. A:Chem. 2015, 406, 204.
(b) Wang, Y. F.; Chen, X. H.; Du, Q. Acta Chim. Sinica 2017, 75, 715(in Chinese). (王一帆, 谌晓洪, 杜泉, 化学学报, 2017, 75, 715.)
[25] Yogish, K.; Sastri, N. V. S. Ind. Eng. Chem. Res. 1988, 27, 909.
[26] Parida, K.; Mishra, K. G.; Dash, S. K. Ind. Eng. Chem. Res. 2012, 51, 2235.
[27] Rajabi, F.; Karimi, N.; Saidi, M. R.; Primo, A.; Varma, R. S.; Luque, R. Adv. Synth. Catal. 2012, 354, 1707.
[28] Tang, T.; Yang, M.; Dong, W.; Tan, L.; Zhang, X.; Zhao, P.; Peng, C.; Wang, G. Microporous Mesoporous Mater. 2015, 215, 199.
[29] Nepak, D.; Srinivas, D. Appl. Catal. A:Gen. 2016, 523, 61.
[30] Wan, Y.; Liang, Q.; Li, Z.; Xu, S.; Hu, X.; Liu, Q.; Lu, D. J. Mol. Catal. A:Chem. 2015, 402, 29.
[31] Chandrasena, R. E.; Vatsis, K.; Coon, M.; Hollenberg, P.; Newcomb, M. J. Am. Chem. Soc. 2004, 126, 115.
[32] Desai, N. C.; Chudasama, J. A.; Karkar, T. J.; Patel, B. Y.; Jadeja, K. A.; Godhani, D. R.; Mehta, J. P. J. Mol. Catal. A:Chem. 2016, 424, 203.
[33] Liu, M. C.; Kong, L. B.; Ma, X. J.; Lu, C.; Li, X. M.; Luo, Y. C.; Kang, L. New. J. Chem. 2012, 36, 1713.
[34] Trinidad, C. S.; Martinez-de la Cruz, A.; Cuellar, E. L. Environ. Sci. Pollut. Res. Int. 2015, 22, 792.
[35] Pelletier, G.; Bechara, W. S.; Charette, A. B. J. Am. Chem. Soc. 2010, 132, 12817.
[36] Lou, J. D.; Gao, C. L.; Li, L.; Fang, Z. G. Monatsh. Chem. 2006, 137, 1071.
/
| 〈 |
|
〉 |