

马烯雌酮及其衍生物的高效合成
收稿日期: 2017-09-05
修回日期: 2017-10-11
网络出版日期: 2017-10-16
Practical Syntheses of Equilin and Its Derivatives
Received date: 2017-09-05
Revised date: 2017-10-11
Online published: 2017-10-16
郑修文 , 马海燕 , 丁凯 . 马烯雌酮及其衍生物的高效合成[J]. 有机化学, 2018 , 38(2) : 464 -470 . DOI: 10.6023/cjoc201709005
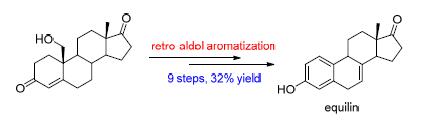
Equilin and its derivatives are important active ingredients of conjugated estrogens, extracted from pregnant horse urine. However, they are still challenge synthetic targets due to the presence of an unstable non-conjugated double bond. Equilin (7), 17β-dihydroequilin (8) and 17α-dihydroequilin (9) were efficiently synthesized from commercially available 19-hydroxyandrost-4-ene-3, 17-dione via retro-aldol aromatization with the overall yields of 32%, 37% and 25%, respectively.
Key words: equilin; conjugated estrogen; aromatization; steroid
[1] (a) Grodin, J. M.; Siiteri, P. K.; Macdonald, P. C. J. Clin. Endocrinol. Metab. 1973, 36, 207.
(b) Longcope, C. Am. J. Obstet. Gynecol. 1971, 111, 778.
(c) Purohit, A.; Tutill, H. J.; Day, J. M.; Chander, S. K.; Lawrence, H. R.; Allan, G. M.; Fischer, D. S.; Vicker, N.; Newman, S. P.; Potter, B. V. L.; Reed, M. J. Mol. Cell. Endocrinol. 2006, 248, 199.
[2] Bhavnani, B. R.; Stanczyk, F. Z. J. Steroid Biochem. Mol. Biol. 2014, 142, 16.
[3] (a) Jing, Y.; Xu, C. -G.; Ding, K.; Lin, J. -R.; Jin, R. -H.; Tian, W. -S. Tetrahedron Lett. 2010, 51, 3242.
(b) Jing, Y. M. S. Thesis, Shanghai Normal University, Shanghai, 2010 (in Chinese). (景羽, 硕士论文, 上海师范大学, 上海, 2010.)
[4] Yue, T.; Li, H. -P.; Ding, K. Tetrahedron Lett. 2016, 57, 4850.
[5] (a) Zderic, J. A.; Bowers, A.; Carpio, H.; Djerassi, C. J. Am. Chem. Soc. 1958, 80, 2596.
(b) Zderic, J. A.; Carpio, H.; Bowers, A.; Djerassi, C. Steroids 1963, 1, 233.
(c) Bagli, J. F.; Morand, P. F.; Wiesner, K.; Gaudry, R. Tetrahedron Lett. 1964, 5, 387.
(d) Stein, R. P.; Buzby, G. C.; Smith, H. Tetrahedron Lett. 1966, (41), 5015.
(e) Stein, R. P.; Buzby, G. C.; Smith, H. Tetrahedron 1970, 26, 1917.
(f) Bailey, E. J.; Gale, A.; Phillipp, G.; Siddons, P. T.; Smith, G., Chem. Commun. 1967, 1253.
(g) Marshall, D. J.; Deghengh, R. Can. J. Chem. 1969, 47, 3127.
[6] Zheng, D. -Q.; Jing, Y.; Zheng, B. -Y.; Ye, Y. -F.; Xu, S.; Tian, W. -S.; Ma, H. -Y.; Ding, K. Tetrahedron 2016, 72, 2164.
[7] (a) Nicolaou, K. C.; Zhong, Y. L.; Baran, P. S. J. Am. Chem. Soc. 2000, 122, 7596.
(b) Nicolaou, K. C.; Montagnon, T.; Baran, P. S.; Zhong, Y. L. J. Am. Chem. Soc. 2002, 124, 2245.
(c) Chen, K.; Liu, C.; Deng, L.; Xu, G. Steroids 2010, 75, 513.
[8] (a) Hogg, J. A.; Lincoln, F. H.; Nathan, A. H.; Hanze, A. R.; Schneider, W. P.; Beal, P. F.; Korman, J. J. Am. Chem. Soc. 1955, 77, 4438.
(b) Allen, G. R.; Weiss, M. J. J. Am. Chem. Soc. 1959, 81, 4968.
(c) Schaub, R. E.; Allen, G. R.; Weiss, M. J., J. Am. Chem. Soc. 1959, 81, 4962.
[9] Larock, R. C.; Hightower, T. R.; Kraus, G. A.; Hahn, P.; Zheng, D. Tetrahedron Lett. 1995, 36, 2423.
[10] Fryszkowska, A.; Peterson, J.; Davies, N. L. Org. Process Res. Dev. 2016, 20, 1520.
[11] Guo, P. -P.; Ding, K. Tetrahedron Lett. 2015, 56, 4096.
[12] (a) Wang, W. -Y.; Jin, H. -A.; Yan, Z. -H.; He, M. -C.; Lin, S.; Tian, W. -S. Tetrahedron Lett. 2017, 58, 3489.
(b) Yan, Z. -H.; Jin, H. -A.; Yu, X. -Q.; Wang, W. -Y.; Tian, W. -S. Chin. J. Org. Chem. 2017, 37, 196(in Chinese). (严兆华, 金红爱, 余信权, 王汪阳, 田伟生, 有机化学, 2017, 37, 196.)(c) Yan, Z. -H.; Tian, H.; Zhao, D. -D.; Jin, H. -A.; Tian, W. -S. Chin. Chem. Lett. 2016, 27, 96.
(d) Yan, Z. -H.; Zhao, D. -D.; Hu, W.; Tian, H.; Li, X. -S.; Tian, W. -S. Chem. J. Chin. Univ. 2015, 36, 2441(in Chinese). (严兆华, 赵冬冬, 胡伟, 田欢, 李小松, 田伟生, 高等学校化学学报, 2015, 36, 2441.)
/
| 〈 |
|
〉 |