

1-苯基-4-取代酞嗪衍生物合成及抗肿瘤活性评价
收稿日期: 2017-07-22
修回日期: 2017-09-29
网络出版日期: 2017-10-20
基金资助
国家自然科学基金(No.81430085)和郑州市科技局科研(No.141PQYJS554)资助项目.
Synthesis and Antitumor Activity of 1-Phenyl-4-substituted Phthalazine Derivatives
Received date: 2017-07-22
Revised date: 2017-09-29
Online published: 2017-10-20
Supported by
Project supported by the National Natural Science Foundation of China (No. 81430085) and the Research Project of Science and Technology Bureau of Zhengzhou City (No. 141PQYJS554).
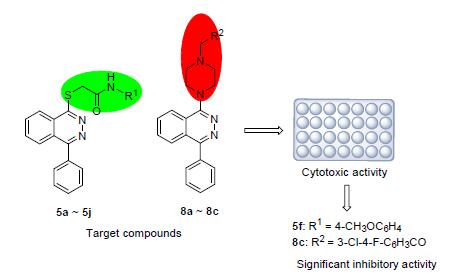
为了找到更有效和更经济的抗肿瘤药物,合成了一系列1-苯基-4-取代酞嗪衍生物,并评估了其体外抗增殖活性.所合成的化合物的结构都通过1H NMR,13 C NMR和HRMS确证.并且通过3-(4,5-二甲基噻唑-2)-2,5-二苯基四氮唑溴盐(MTT)法评估了目标化合物对四种人类癌细胞株的抗肿瘤活性.结果表明:一些化合物具有良好的抗肿瘤活性,特别是N-(4-甲氧基苯基)-2-((4-苯基酞嗪-1-基)硫基)乙酰胺(5f)和N-(3-氯-4-氟苯基)-2-(4-(4-苯基酞嗪-1-基)哌嗪-1-基)乙酰胺(8c)表现出了更好的抗肿瘤活性,对人类食管癌细胞的活性优于5-氟尿嘧啶.IC50值分别为8.13和9.31 μmol·L-1.
辛景超 , 栗娜 , 马启胜 , 李二冬 , 孟祥川 , 可钰 , 刘宏民 , 张秋荣 . 1-苯基-4-取代酞嗪衍生物合成及抗肿瘤活性评价[J]. 有机化学, 2018 , 38(2) : 451 -456 . DOI: 10.6023/cjoc201707025
In order to find more efficient and economical antitumor drugs, a series of 1-phenyl-4-substituted phthalazine derivatives were synthesized and evaluated for antiproliferative activity in vivo. The structures of the synthesized compounds were confirmed by 1H NMR, 13C NMR and HRMS. The antitumor activity of the target compounds was performed against four cancer cell lines by 3-(4, 5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2-H-tetrazolium bromide (MTT). The results showed that some compounds had a good antitumor activity, especially, N-(4-methoxyphenyl)-2-((4-phenylphthalazin-1-yl) thio) acetamide (5f) and N-(3-chloro-4-fluorophenyl)-2-(4-(4-phenylphthalazin-1-yl) piperazin-1-yl) acetamide (8c), exhibited better antitumor activities with IC50 values of 8.13 and 9.31 μmol•L-1 against the human esophageal cancer cells, which were superior to 5-fuorouracil.

Key words: synthesis; phthalazine derivative; antitumor activity
[1] For statistical information about cancer, see:World Health Organization. http://www. who. int/mediacentre/factsheets/fs297/zh/.
[2] Curtin, N. Biochem. Soc. Trans. 2014, 42, 82.
[3] Bryant, H. E.; Helleday, T. Nucleic Acids Res. 2006, 34, 1685.
[4] Bryant, H. E.; Schultz, N.; Thomas, H. D.; Parker, K. M.; Flower, D.; Lopez, E.; Kyle, S.; Meuth, M.; Curtin, N. J.; Helleday, T. Nature 2005, 434, 913.
[5] Dillon, K. J.; Smith, G. C.; Martin, N. M. J. Biomol. Screening 2003, 8, 347.
[6] Ye, Q. Y. J. Int. Oncol. 2007, 34, 579(in Chinese). (叶泉英, 国际肿瘤学杂志, 2007, 34, 579.)
[7] Piatnitski, E. L.; Duncton, M. A. J.; Kiselyov, A. S.; Katoch-Rouse, R.; Sherman, D.; Milligan, D. L.; Balagtas, C.; Wong, W. C.; Kawakami, J. Bioorg. Med. Chem. Lett. 2005, 15, 4696.
[8] Kiselyov, A. S.; Semenov, V. V.; Milligan, D. Chem. Biol. Drug Des. 2006, 68, 308.
[9] Kiselyov, A. S.; Semenov, V. V.; Milligan, D. Chem. Biol. Drug Des. 2006, 68, 308.
[10] Zhang, S. L.; Zhao, Y. F.; Liu, Y. J.; Chen, D.; Lan, W. H.; Zhao, Q. L.; Dong, C. C.; Xia, L.; Gong, P. Eur. J. Med. Chem. 2010, 45, 3504.
[11] Liu, Y. J.; Zhang, S. L.; Li, Y. Wang, J. Q.; Song, Y.; Gong, P. Arch. Pharm. Chem. Life Sci. 2012, 345, 287.
[12] Wang, C. J.; Cao, Q. P.; Yang, H.; Song, P. P.; Xue, D. Q.; Cui, F.; Gu, Y. F.; Zhang, X. S.; Tian, Y. N.; Zhang, Q. R.; Liu, H. M. Chin. J. Org. Chem. 2016, 36, 1626(in Chinese). (王超杰, 曹钦坡, 杨慧, 宋攀攀, 薛登启, 崔飞, 顾一飞, 张孝松, 田亚楠, 张秋荣, 刘宏民, 有机化学, 2016, 36, 1626.)
[13] Hemdan, M. M.; Taha, S. M.; Gabr, A. M.; Elkady, M. Y. J. Chem. Res. 2010, 34, 102.
[14] Grasso, S.; Sarro, G. D.; Sarro, A. D.; Micale, N.; Zappalà, M.; Puja, G.; Baraldi, M.; Micheli, C. D. J. Med. Chem. 2000, 43, 851.
[15] Mertens, M. D.; Pietsch, M.; Schnakenburg, G.; Gütschow, M. J. Org. Chem. 2013, 45, 8966.
[16] Zhang, S. L.; Zhao, Y. F.; Liu Y.; Chen, D.; Lan, W. H.; Zhao, Q. L.; Dong, C. C.; Xia, L.; Gong, P. Eur. J. Med. Chem. 2010, 45, 3504.
[17] Zhang, Q. R.; Xue, D. Q.; He, P.; Shao, K. P.; Chen, P. J.; Gu, Y. F.; Ren, J. L.; Shan, L. H.; Liu, H. M. Bioorg. Med. Chem. Lett. 2014, 24, 1236.
[18] Wu, Y.; Sun, L. P.; Ma, L. X.; Chen, J.; Song, M. X.; Cui, X.; Piao, H. R. Chem. Biol. Drug Des. 2013, 81, 591.
[19] Abou-Seri, S. M.; Eldehna, W. M.; Ali, M. M.; Abou El Ella, D. A. Eur. J. Med. Chem. 2016, 107, 165.
[20] Zhou, Z. C.; Shu, W. Y. J. Cent. South Univ. 2002.
/
| 〈 |
|
〉 |