

含喹唑啉-4-酮片段的新型1,2,4-三唑酰腙类衍生物的合成及抗菌活性
收稿日期: 2017-08-24
修回日期: 2017-09-28
网络出版日期: 2017-10-20
基金资助
国家自然科学基金(No.21362003)和贵州省农业攻关(No.20093010)资助项目.
Synthesis and Antimicrobial Activities of Novel 1,2,4-Triazole-acyl-hydrazone Derivatives Containing the Quinazolin-4-one Moiety
Received date: 2017-08-24
Revised date: 2017-09-28
Online published: 2017-10-20
Supported by
Project supported by the National Natural Science Foundation of China (No. 21362003) and the Agricultural Research Projects of Guizhou Province (No. 20093010).
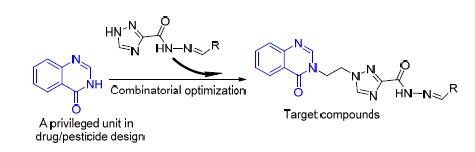
通过三唑酰肼和芳醛的缩合反应,合成了20个含喹唑啉酮-4-酮片段的新型1,2,4-三唑酰腙类化合物,利用核磁共振氢谱、碳谱和高分辨质谱对它们的结构进行了表征.体外抗菌测试表明,绝大部分目标化合物对水稻白叶枯病菌和柑橘溃疡病菌都表现出良好的抑制活性,其中N'-(2-甲氧基亚苄基)-1-(2-(4-氧代喹唑啉-3(4H)-基)乙基)-1H-1,2,4-三唑-3-酰腙(7q)在100 μg/mL下对上述两种病菌的抑制率均达100%.此外,1-(2-(4-氧代喹唑啉-3(4H)-基)乙基)-N'-(4-(三氟甲基)亚苄基)-1H-1,2,4-三唑-3-酰腙(7i)、N'-(2-溴亚苄基)-1-(2-(4-氧代喹唑啉-3(4H)-基)乙基)-1H-1,2,4-三唑-3-酰腙(7j)、N'-(4-溴亚苄基)-1-(2-(4-氧代喹唑啉-3(4H)-基)乙基)-1H-1,2,4-三唑-3-酰腙(7l)和N'-(4-甲基亚苄基)-1-(2-(4-氧代喹唑啉-3(4H)-基)乙基)-1H-1,2,4-三唑-3-酰腙(7o)在50 μg/mL下对番茄灰霉病菌的抑制率均超过55%.
关键词: 1,2,4-三唑酰腙; 喹唑啉-4-酮; 合成; 抗菌活性
杜欢 , 范治江 , 杨岚 , 鲍小平 . 含喹唑啉-4-酮片段的新型1,2,4-三唑酰腙类衍生物的合成及抗菌活性[J]. 有机化学, 2018 , 38(2) : 531 -538 . DOI: 10.6023/cjoc201708051
A total of twenty novel 1, 2, 4-triazole-acylhydrazone derivatives containing the quinazolin-4-one moiety were synthesized via the condensation reaction of triazole hydrazide with various aromatic aldehydes, and fully characterized by 1H NMR, 13C NMR and HRMS spectra. Antimicrobial assays in vitro indicated that most of the target compounds exhibited good antibacterial activities against the pathogenic phytobacteria Xanthomonas oryzae pv. oryzae(Xoo) and Xanthomonas axonopodis pv. citri(Xac). Notably, N'-(2-methoxybenzylidene)-1-(2-(4-oxoquinazolin-3(4H)-yl) ethyl)-1H-1, 2, 4-triazole-3-acylhy-drazone)(7q) displayed the inhibition rate of 100% against the above two bacteria at 100 μg/mL. Additionally, 1-(2-(4-oxo-quinazolin-3(4H)-yl) ethyl)-N'-(4-(trifluoromethyl) benzylidene)-1H-1, 2, 4-triazole-3-acylhydrazone (7i), N'-(2-bromobenzyli-dene)-1-(2-(4-oxoquinazolin-3(4H)-yl) ethyl)-1H-1, 2, 4-triazole-3-acylhydrazone (7j), N'-(4-bromobenzylidene)-1-(2-(4-oxo-quinazolin-3(4H)-yl) ethyl)-1H-1, 2, 4-triazole-3-acylhydrazone (7l), and N'-(4-methoxybenzylidene)-1-(2-(4-oxoquinazolin-3(4H)-yl) ethyl)-1H-1, 2, 4-triazole-3-acylhydrazone (7o) were found to possess the inhibition rate of >55% against the fungus Botrytis cinerea Pers. at 50 μg/mL.

[1] Sun, X.; Cao, Y.; Yang, Z.; Xu, C.; Li, X.; Wang, S.; Zhang, Q. Plant J. 2004, 37, 517.
[2] Graham, J. H.; Gottwald, T. R.; Cubero, J.; Achor, D. S. Mol. Plant Pathol. 2004, 5, 1.
[3] Salanoubat M.; Genin S.; Artiguenave F.; Gouzy J.; Mangenot S.; Arlat M.; Billaultk A.; Brottier P.; Camus J. C.; Cattolico L.; Chandler M.; Choisne N.; Claudel-Renard C.; Cunnac S.; Demange, N.; Gaspin C.; Lavie M.; Moisan A.; Robert C.; Saurin W.; Schiex T.; Siguier P.; Thébault P.; Whalen M.; Wincker P.; Levy M.; Weissenbach J.; Boucher C. A. Nature 2002, 415, 497.
[4] Ryan R. P.; Vorhölter F. J.; Potnis N.; Jones J. B.; Van Sluys M. A.; Bogdanove A. J.; Dow J. M. Nat. Rev. Microbiol. 2011, 9, 344.
[5] Li J.; Wang N. PLoS One 2011, 6, e21804.
[6] Chang, Y. H.; Zhang, L.; Liu Y. Z.; Chen, Z. Y. Jiangsu Agric. Sci. 2012, 40, 89(in Chinese). (常有宏, 张磊, 刘邮洲, 陈志谊, 江苏农业科学, 2012, 40, 89.)
[7] Sun, N.; Du, R. L.; Zheng, Y. Y.; Huang, B. H.; Guo, Q.; Zhang, R. F.; Wong, K. Y.; Lu, Y. J. Eur. J. Med. Chem. 2017, 135, 1.
[8] Shavit, M.; Pokrovskaya, V.; Belakhov, V.; Baasov, T. Bioorg. Med. Chem. 2017, 25, 2917.
[9] Zhang, F.; Wen, Q.; Wang, S. F.; Shahla, K. B.; Yang, Y. S.; Liu, J. J.; Zhang, W. M.; Zhu, H. L. Bioorg. Med. Chem. Lett. 2014, 24, 90.
[10] Somagond, S. M.; Kamble, R. R.; Kattimani, P. P.; Joshi, S. D.; Dixit, S. R. Heterocycl. Commun. 2017, 23, 317.
[11] Kulabas, N.; Tatar, E.; Özakpinar, Ö. B.; Özsavci, D.; Pannecouque, C.; Clercq, E. D.; Küçükgüzel, I. Eur. J. Med. Chem. 2016, 121, 58.
[12] Basaran, E.; Karakucuk-Iyidogan, A.; Schols, D.; Oruc-Emre, E. E. Chirality 2016, 28, 495.
[13] Xiong, Q.; Liu, J.; Lin, X.; Bao, X. Chin. J. Org. Chem. 2012, 32, 1951(in Chinese). (熊启中, 刘军虎, 林选福, 鲍小平, 有机化学, 2012, 32, 1951.)
[14] Che, Z.; Zhang, S.; Shao, Y.; Fan, L.; Xu, H.; Yu, X.; Zhi, X. Y.; Yao, X. J.; Zhang, R. J. Agric. Food Chem. 2013, 61, 5696.
[15] Wang, X.; Yin, J.; Shi, L.; Zhang, G. P.; Song, B. A. Eur. J. Med. Chem. 2014, 77, 65.
[16] Zhang, J.; Liu, J.; Ma, Y.; Ren, D.; Cheng, P.; Zhao, J. W.; Zhang, F.; Yao, Y. Bioorg. Med. Chem. Lett. 2016, 26, 2273.
[17] Ding, P. P.; Gao, M.; Mao, B. B.; Cao, S. L.; Liu, C. H.; Yang, C. R.; Li, Z. F.; Liao, J.; Zhao, H.; Li, Z.; Li, J.; Wang, H.; Xu, X. Eur. J. Med. Chem. 2016, 108, 364.
[18] Chen, M.; Li, P.; Hu, D.; Zeng, S.; Li, T.; Jin, L.; Xue, W.; Song, B. Bioorg. Med. Chem. Lett. 2016, 26, 168.
[19] Zhu, S. S.; Lu, J. R.; Xin, C. W.; Lu, B. W.; Bao, X. R.; Zou, M.; Liu, Q. Chem. J. Chin. Univ. 2010, 31, 2228(in Chinese). (朱姗姗, 卢俊瑞, 辛春伟, 卢博为, 鲍秀荣, 邹敏, 刘倩, 高等学校化学学报, 2010, 31, 2228.)
[20] Zhang, M.; Lu, J.; Xin, C.; Liu, F.; Wang, J.; Li, H.; Wei, R.; Bao, X. Chin. J. Org. Chem. 2009, 29, 1645(in Chinese). (张明, 卢俊瑞, 辛春伟, 刘芳, 王菁菁, 李红姬, 魏荣宝, 鲍秀荣, 有机化学, 2009, 29, 1645.)
[21] Liu, J.; Liu, Y.; Jian, J.; Bao, X. Chin. J. Org. Chem. 2013, 33, 370(in Chinese). (刘军虎, 刘勇, 蹇军友, 鲍小平, 有机化学, 2013, 33, 370.)
[22] Yan, B.; Lv, X.; Du, H.; Bao, X. Chin. J. Org. Chem. 2016, 36, 207(in Chinese). (闫柏任, 吕新阳, 杜欢, 鲍小平, 有机化学, 2016, 36, 207.)
[23] Yan, B. R.; Lv, X. Y.; Du, H.; Gao, M. N.; Huang, J.; Bao, X. P. Chem. Pap. 2016, 70, 983.
[24] Zhang, G.; Fu, X.; Peng, X.; Li, X.; Chen, J. J. Chem. Res. 2013, 37, 730.
[25] Yao, Y. P.; Dai, F. Y.; Dong, K. K.; Mao, Q.; Wang, Y. L.; Chen, T. J. Chem. Res. 2011, 35, 4.
[26] Okuda, K.; Ohtomo, H.; Tagata, T.; Hirota, T.; Sasaki, K. Synth. Commun. 2011, 41, 812.
[27] Yang, L.; Bao, X. RSC Adv. 2017, 7, 34005.
[28] Fan, Z.; Shi, Z.; Zhang, H.; Liu, X.; Bao, L.; Ma, L.; Zuo, X.; Zheng, Q.; Mi, N. J. Agric. Food Chem. 2009, 57, 4279.
[29] Chen, C. J.; Song, B. A.; Yang, S.; Xu, G. F.; Bhadury, P. S.; Jin, L. H.; Hu, D. Y.; Li, Q. Z.; Liu, F.; Xue, W.; Lu, P.; Chen, Z. Bioorg. Med. Chem. 2007, 15, 3981.
/
| 〈 |
|
〉 |