

Cudratricusxanthone B的全合成
收稿日期: 2017-08-30
修回日期: 2017-09-21
网络出版日期: 2017-10-24
基金资助
国家自然科学基金(Nos.21472025,21172044,81222045,21372049)资助项目.
Total Synthesis of Cudratricusxanthone B
Received date: 2017-08-30
Revised date: 2017-09-21
Online published: 2017-10-24
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21472025, 21172044, 81222045, 21372049).
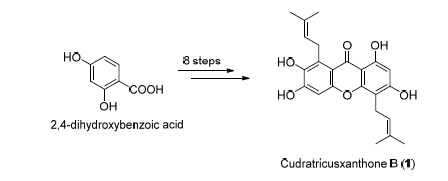
以价廉易得的2,4-二羟基苯甲酸为起始原料,通过Claisen重排和选择性脱甲基等8步反应,以3.1%的总收率首次完成了具有生物活性的异戊烯基氧杂蒽酮类天然产物cudratricusxanthone B(1)的全合成,为该化合物进一步的生物活性研究奠定了物质基础.同时还合成得到另一氧杂蒽酮化合物2,证实文献报道的氧杂蒽酮类天然产物staudtiixanthone D结构鉴定有误.
关键词: Cudratricusxanthone B; 异戊烯基氧杂蒽酮; Claisen重排; 全合成
周鹏飞 , 侯爱君 , 王洋 . Cudratricusxanthone B的全合成[J]. 有机化学, 2018 , 38(1) : 156 -161 . DOI: 10.6023/cjoc201708060
The efficient total synthesis of cudratricusxanthone B (1), a biologically interesting natural isoprenylated xanthone, has been achieved for the first time starting from commercially available 2,4-dihydroxybenzoic acid via a linear reaction sequence of 8 steps with the overall yield of 3.1%, wherein Claisen rearrangement and demethylation with AlCl3/pyridine are used as key reactions. This work definitely laid the foundation for the further pharmacological study of this natural compound. Meanwhile, another xanthone (2) has been synthesized, which proved the reported structure of the natural xanthone staudtiixanthone D to be wrong.

[1] Zou, Y. S.; Hou, A. J.; Zhu, G. F.; Chen, Y. F.; Sun, H. D.; Zhao, Q. S. Bioorg. Med. Chem. 2004, 12, 1947.
[2] Zhang, Z.; Wu, H. J.; Pi, E. H.; Zhang, T.; Yuan, W. Y.; Hou, A. J.; Liu Q. H. World Clin. Drugs 2009, 30, 601(in Chinese).(张志, 吴海健, 皮恩浩, 张瑱, 袁文越, 侯爱君, 刘全海, 世界临床药物, 2009, 30, 601.)
[3] Ngoupayo, J.; Tabopda, T. K.; Ali, M. S. Bioorg. Med. Chem. 2009, 17, 5688.
[4] (a) Masters, K. S.; Brase, S. Chem. Rev. 2012, 112, 3717.
(b) Sousa; M. E.; Pinto; M. M. M. Curr. Med. Chem. 2005, 12, 2447.
(c) Iikubo, K.; Ishikawa, Y.; Ando, N.; Umezawa, K.; Nishiyam, S. Tetrahedron Lett. 2002, 43, 291.
(d) Hintermann, L.; Masuo, R.; Suzuki, K. Org. Lett. 2008, 10, 4859.
(e) Lin, S.; Koh, J. J.; Aung, T. T.; Lim, F.; Li, J.; Zou, H.; Wang, L.; Lakshminarayanan, R.; Verma, C.; Wang, Y.; Tan, D. T. H.; Cao, D.; Beuerman, R. W.; Ren, Li.; Liu, S. J. Med. Chem. 2017, 60, 1362.
(f) Lewis, J. R. Proc. Chem. Soc. 1963, 373.
(g) Yamazaki, S. Org. Lett. 1999, 1, 2129.
[5] Keana, J. F. W.; Guzikowski, A. P.; Nogales, D. F.; Cai, S. X. US 5476933, 1995[Chem. Abstr. 1995, 124, 232271].
[6] Kumar, S.; Reddy L, C. S.; Kumar, Y.; Kumar, A.; Singh, B. K.; Kumar, V.; Malhotra, S.; Pandey, M. K.; Jain, R.; Thimmulappa, R.; Sharma, S. K.; Prasad, A. K.; Biswal, S.; Van der Eycken, E.; DePass, A. L.; Malhotra, S. V.; Ghosh, B.; Parmar, V. S. Arch. Pharm. Chem. Life Sci. 2012, 345, 368.
[7] Oida, S.; Ohashi, Y.; Ohki, E. Chem. Pharm. Bull. 1973, 21, 528.
[8] Wu, Z.; Wei, G.; Lian, G.; Yu, B. J. Org. Chem. 2010, 75, 5725.
[9] Tisdale, E. J.; Slobodov, I.; Theodorakis, E. A. Org. Biomol. Chem. 2003, 1, 4418.
[10] Sun, H.; Chen, F.; Wang, X.; Liu, Z.; Yang, Q.; Zhang, X.; Zhu, J.; Qiang, L.; Guo, Q.; You, Q. Eur. J. Med. Chem. 2012, 51, 110.
[11] Kapingu, M. C.; Magadula, J. J. Nat. Prod. Commun. 2008, 3(9), 1501.
[12] Gokaraju, G. R.; Gokaraju, R. R.; Golakoti, T.; Somepalli, V.; Bhupathiraju, K. WO 2009093259, 2009[Chem. Abstr. 1983, 151, 198136].
[13] Fache, M.; Darroman, E.; Besse, V.; Auvergne, R.; Caillol, S.; Boutevin, B. Green Chem. 2014, 16, 1987.
[14] Shinoda, J.; Sato, D.; Kawagoye, M. Yakugaku Zasshi 1932, 52, 766.
[15] Hardegger, E.; Steiner, K.; Widmer, E.; Corrodi, H.; Schmidt, T.; Knoepfel, H. P.; Rieder, W.; Meyer, H. J.; Kugler, F.; Gempeler, H. Helv. Chim. Acta 1964, 47, 1996.
[16] Evans, J. C.; Klix, R. C.; Bach, R. D. J. Org. Chem. 1988, 53, 5519.
[17] Ueno, A. Yakugaku Zasshi 1962, 82, 1482.
/
| 〈 |
|
〉 |