

银催化9-联烯嘌呤与氟代双(苯磺酰基)甲烷的单氟甲基化反应
收稿日期: 2017-11-01
修回日期: 2017-11-19
网络出版日期: 2017-11-21
基金资助
国家自然科学基金(Nos.U1604283,21402041)、河南省科技创新杰出人才(No.164200510008)、中国博士后科学基金(No.2016M592293)、河南省高校创新团队(No.15IRTSTHN003)和高等学校学科创新引智计划(111计划,No.D17007)资助项目.
Ag-Catalyzed Monofluoromethylation of Purin-9-yl Allenes with Fluorobis(phenylsulfonyl)methane
Received date: 2017-11-01
Revised date: 2017-11-19
Online published: 2017-11-21
Supported by
Project supported by the National Natural Science Foundation of China (Nos. U1604283, 21402041), the Plan for Scientific Innovation Talent of Henan Province (No. 164200510008), the China Postdoctoral Science Foundation Funded Project (No. 2016M592293), the Program for Innovative Research Team in Science, Technology in University of Henan Province (No. 15IRTSTHN003) and the Program of Introducing Talents of Discipline to Universities (111 Project, No. D17007).
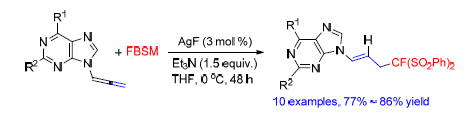
以氟代双(苯磺酰基)甲烷为氟化试剂,AgF(3 mol%)为催化剂,实现了9-联烯嘌呤的单氟甲基化反应,并在不同取代基的9-联烯嘌呤底物中均表现出了较好的收率.单氟甲基化反应具有较高的化学选择性和E-选择性.同时,这种方法为合成含氟嘌呤化合物提供了一种有效的途径.
关键词: 单氟甲基化反应; 9-联烯嘌呤; 氟代双(苯磺酰基)甲烷
郭真 , 谢明胜 , 韩瑞杰 , 渠桂荣 , 郭海明 . 银催化9-联烯嘌呤与氟代双(苯磺酰基)甲烷的单氟甲基化反应[J]. 有机化学, 2018 , 38(1) : 112 -117 . DOI: 10.6023/cjoc201711001
The monofluoromethylation of purin-9-yl allenes with fluorobis(phenylsulfonyl)methane has been achieved. With AgF (3 mol%) as the catalyst, the fluorobis(phenylsulfonyl)methylated adducts could be afforded in excellent yields. The monofluoromethylation exhibited high chemoselectivities and E-selectivies. Meanwhile, the monofluoromethylation of purin-9-yl allenes with fluorobis(phenylsulfonyl)methane provided a useful route to construct fluorinated acyclic nucleoside analogues.

[1] (a) Smart, B. E. J. Fluorine Chem. 2001, 109, 3.
(b) Kirsch, P. Modern Fluoroorganic Chemistry, Wiley-VCH, Weinheim, Germany, 2004.
(c) Kirk, K. L. J. Fluorine Chem. 2006, 127, 1013.
(d) Li, Y.; Ni, C.; Liu, J.; Zhang, L.; Zheng, J.; Zhu, L.; Hu, J. Org. Lett. 2006, 8, 1693.
(e) Bégué, J.-P.; Bonnet-Delpon, D. J. Fluorine Chem. 2006, 127, 992.
(f) Prakash, G. K. S.; Hu, J. Acc. Chem. Res. 2007, 40, 921.
(g) Müller, K.; Faeh, C.; Diederich, F. Science 2007, 317, 1881.
(h) Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 320.
(i) Hu, J.; Zhang, W.; Wang, F. Chem. Commun. 2009, 7465.
(j) Liao, F.-M.; Yu, J.-S.; Zhou, J. Chin. J. Org. Chem. 2017, 37, 2175(in Chinese).(廖富民, 余金生, 周剑, 有机化学, 2017, 37, 2175.)
(k) Liao, F.-M.; Cao, Z.-Y.; Yu, J.-S.; Zhou, J. Angew. Chem., Int. Ed. 2017, 56, 2459.
[2] Fukuzumi, T.; Shibata, N.; Sugiura, M.; Yasui, H.; Nakamura, S.; Toru, T. Angew. Chem., Int. Ed. 2006, 45, 4973
[3] Ni, C.; Li, Y.; Hu, J. J. Org. Chem. 2006, 71, 6829.
[4] Prakash, G. K. S.; Chacko, S.; Alconcel, S.; Stewart, T.; Mathew, T.; Olah, G. A. Angew. Chem., Int. Ed. 2007, 46, 4933.
[5] (a) Furukawa, T.; Goto, Y.; Kawazoe, J.; Tokunaga, E.; Naka-mura, S.; Yang, Y.; Du, H.; Kakehi, A.; Shiro, M.; Shibata, N. Angew. Chem., Int. Ed. 2010, 49, 1642.
(b) Prakash, G. K. S.; Shao, N.; Zhang, Z.; Ni, C.; Wang, F.; Haiges, R.; Olah, G. A. J. Fluorine Chem. 2012, 133, 27.
(c) Shen, X.; Miao, W.; Ni, C.; Hu, J. Angew. Chem., Int. Ed. 2014, 53, 775.
[6] (a) Liu, W.-B.; Zheng, S.-C.; He, H.; Zhao, X.-M.; Dai, L.-X.; You, S.-L. Chem. Commun. 2009, 6604.
(b) Furukawa, T.; Kawazoe, J.; Zhang, W.; Nishimine, T.; Tokunaga, E.; Matsumoto, T.; Shiro, M.; Shibata, N. Angew. Chem., Int. Ed. 2011, 50, 9684.
(c) Yang, W.; Wei, X.; Pan, Y.; Lee, R.; Zhu, B.; Liu, H.; Yan, L.; Huang, K.-W.; Jiang, Z.; Tan, C.-H. Chem. Eur. J. 2011, 17, 8066.
[7] (a) Shen, X.; Zhang, L.; Zhao, Y.; Zhu, L.; Li, G.; Hu, J. Angew. Chem., Int. Ed. 2011, 50, 2588.
(b) Ma, H.; Matsuzaki, K.; Yang, Y.-D.; Tokunaga, E.; Nakane, D.; Ozawa, T.; Masuda, H.; Shibata, N. Chem. Commun. 2013, 49, 11206.
(c) Shen, X.; Ni, C.; Hu, J. Chin. J. Chem. 2013, 31, 878.
(d) Mizuta, S.; Shibata, N.; Goto, Y.; Furukawa, T.; Nakamura, S.; Toru, T. J. Am. Chem. Soc. 2007, 129, 6394.
(e) Prakash, G. K. S.; Gurung, L.; Jog, P. V.; Tanaka, S.; Thomas, T. E.; Ganesh, N.; Haiges, R.; Mathew, T.; Olah, G. A. Chem. Eur. J. 2013, 19, 3579.
[8] (a) Furukawa, T.; Shibata, N.; Mizuta, S.; Nakamura, S.; Toru, T.; Shiro, M. Angew. Chem., Int. Ed. 2008, 47, 8051.
(b) Zhang, S.; Zhang, Y.; Ji, Y.; Li, H.; Wang, W. Chem. Commun. 2009, 4886.
(c) Alba, A.-N.; Companyó, X.; Moyano, A.; Rios, R. Chem. Eur. J. 2009, 15, 7035.
(d) Moon, H. W.; Cho, M. J.; Kim, D. Y. Tetrahedron Lett. 2009, 50, 4896.
(e) Ullah, F.; Zhao, G.-L.; Deiana, L.; Zhu, M.; Dziedzic, P.; Ibrahem, I.; Hammar, P.; Sun, J.; Córdova, A. Chem. Eur. J. 2009, 15, 10013.
[9] Ni, C.; Zhang, L.; Hu, J. J. Org. Chem. 2008, 73, 5699.
[10] hen, X.; Zhang, W.; Zhang, L.; Luo, T.; Wan, X.; Gu, Y.; Hu, J. Angew. Chem., Int. Ed. 2012, 51, 6966.
[11] Ogasawara, M.; Murakami, H.; Furukawa, T.; Takahashi, T.; Shibata, N. Chem. Commun. 2009, 7366.
[12] (a) Baszczyňski, O.; Zaneba, Z. Med. Res. Rev. 2013, 33, 1304.
(b) Jindrich, J.; Holý, A.; Dvoráková, H. Collect. Czech. Chem. Commun. 1993, 58, 1645.
(c) Kiesewetter, D. O.; Knudson, K.; Collins, M.; Srinivasula, S.; Lim, E.; Mascio, M. D. J. Labelled Compd. Radiopharm. 2008, 51, 187.
[13] (a) Liang, L.; Xie, M.-S.; Wang, H.-X.; Niu, H.-Y.; Qu, G.-R.; Guo, H.-M. J. Org. Chem. 2017, 82, 5966.
(b) Liang, L.; Xie, M.-S.; Qin, T.; Zhu, M.; Qu, G.-R.; Guo, H.-M. Org. Lett. 2017, 19, 5212.
(c) Sun, H.-L.; Chen, F.; Xie, M.-S.; Guo, H.-M.; Qu, G.-R.; He, Y.-M.; Fan, Q.-H. Org. Lett. 2016, 18, 2260.
(d) Zhang, D.-J.; Xie, M.-S.; Qu, G.-R.; Gao, Y.-W.; Guo, H.-M. Org. Lett. 2016, 18, 820.
(e) Xie, M.-S.; Wang, Y.; Li, J.-P.; Du, C.; Zhang, Y.-Y.; Hao, E.-J.; Zhang, Y.-M.; Qu, G.-R.; Guo, H.-M. Chem. Commun. 2015, 51, 12451.
(f) Niu, H.-Y.; Du, C.; Xie, M.-S.; Wang, Y.; Zhang, Q.; Qu, G.-R.; Guo, H.-M. Chem. Commun. 2015, 51, 3328.
(g) Wei, T.; Xie, M.-S.; Qu, G.-R.; Niu, H.-Y.; Guo, H.-M. Org. Lett. 2014, 16, 900.
[14] For reviews about synthesis of acyclic nucleoside analogues, see:(a) Xie, M.-S.; Niu, H.-Y.; Qu, G.-R.; Guo, H.-M. Tetrahedron Lett. 2014, 55, 7156.
(b) Guo, H.-M.; Wu, S.; Niu, H.-Y.; Song, G.; Qu, G.-R. In Chemical Synthesis of Nucleoside Analogues, Ed.:Merino, P., John Wiley & Sons, Hoboken, New Jersey, 2013, p. 103.
/
| 〈 |
|
〉 |