

在含水介质中钯高效催化咪唑并[1,2-a]吡啶类化合物与芳基氯代物的碳氢芳基化反应
收稿日期: 2017-10-24
修回日期: 2017-11-27
网络出版日期: 2017-11-28
基金资助
国家自然科学基金(No.21702191)、河南省科技攻关项目(No.172102210555)和河南省博士后科研(No.2014003)资助项目.
Efficient Pd-Catalyzed Direct C-H Bond Arylation of Imidazo-[1, 2-a]pyridines with Aryl Chlorides in Aqueous Medium
Received date: 2017-10-24
Revised date: 2017-11-27
Online published: 2017-11-28
Supported by
Project supported by the National Natural Science Foundation of China (No. 21702191), the Scientific and Technological Project of Henan Province (No. 172102210555) and the Postdoctoral Science Foundation of Henan Province (No. 2014003).
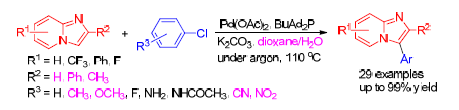
3-芳基咪唑并[1,2-a]吡啶骨架广泛存在于药物结构中,在药物学方面具有重要的地位及在材料化学、有机化学等方面也具有潜在的应用价值.在含水介质中,利用醋酸钯催化咪唑并[1,2-a]吡啶及其衍生物与芳基/杂芳基氯代物的碳氢芳基化反应,简便、高效地合成系列3-芳基咪唑并[1,2-a]吡啶类化合物,并以较好至优秀的收率获得芳基化产品.该方法采用廉价易得的芳基/杂芳基氯代物和咪唑并[1,2-a]吡啶类化合物为偶联反应的底物,且底物的范围能够拓展至缺电子、富电子的芳基氯代物和杂芳基氯代物及多种基团取代的咪唑并[1,2-a]吡啶类化合物.
关键词: 碳氢芳基化; 咪唑并[1,2-a]吡啶; 3-芳基咪唑并[1,2-a]吡啶; 芳基/杂芳基氯代物; 含水介质
穆兵 , 李敬亚 , 邹大鹏 , 吴豫生 , 常俊标 , 吴养洁 . 在含水介质中钯高效催化咪唑并[1,2-a]吡啶类化合物与芳基氯代物的碳氢芳基化反应[J]. 有机化学, 2018 , 38(1) : 95 -102 . DOI: 10.6023/cjoc201710029
An efficient and practical protocol for palladium-catalyzed direct C—H bond arylation of imidazo[1,2-a]pyridines with cheap aryl/heteroaryl chlorides has been developed. Various imidazo[1,2-a]pyridines with electron-neutral, electron-poor, electron-rich, even sterically hindered aryl chlorides and heteroaryl chlorides were successfully applied to the reaction in aqueous medium to achieve the 3-arylimidazo[1,2-a]pyridines in mostly good to excellent yields, thus representing a signi?- cant advancement in the implementation of the direct C—H bond arylation of imidazo[1,2-a]pyridines with aryl chlorides.

[1] Bagdi, A. K.; Santra, S.; Monir, K.; Hajra, A. Chem. Commun. 2015, 51, 1555.
[2] Moraski, G. C.; Markley, L. D.; Hipskind, P. A.; Boshoff, H.; Cho, S.; Franzblau, S. G.; Miller, M. J. ACS Med. Chem. Lett. 2011, 2, 466.
[3] Egner, U.; Gerbling, K. P.; Hoyer, G. A.; Krüger, G.; Wegner, P. Pestic. Sci. 1996, 47, 145.
[4] Bae, J. S.; Lee, D. W.; Lee, D. H.; Jeong, D. S. WO 2007011163, 2007[Chem. Abstr. 2007, 146, 193485].
[5] El-Sayed, W. M.; Hussin, W. A.; Al-Faiyz, Y. S.; Ismail, M. A. Eur. J. Pharmacol. 2013, 715, 212.
[6] Lacerda, R. B.; de Lima, C. K. F.; da Silva, L. L.; Romeiro, N. C.; Miranda, A. L. P.; Barreiro, E. J.; Fraga, C. A. M. Bioorg. Med. Chem. 2009, 17, 74.
[7] Shukla, N. M.; Salunke, D. B.; Yoo, E.; Mutz, C. A.; Balakrishna, R.; David, S. A. Bioorg. Med. Chem. 2012, 20, 5850.
[8] Ismail, M. A.; Arafa, R. K.; Wenzler, T.; Brun, R.; Tanious, F. A.; Wilson, W. D.; Boykin, D. W. Bioorg. Med. Chem. 2008, 16, 683.
[9] Véron, J. B.; Allouchi, H.; Enguehard-Gueiffier, C.; Snoeck, R.; Andrei, G.; De Clercq, E.; Gueiffier, A. Bioorg. Med. Chem. 2008, 16, 9536.
[10] Kaminski, J. J.; Doweyko, A. M. J. Med. Chem. 1997, 40, 427.
[11] Rival, Y.; Grassy, G.; Taudon, A.; Ecalle, R. Eur. J. Med. Chem. 1991, 26, 13.
[12] Enguehard-Gueiffier, C.; Musiu, S.; Henry, N.; Véron, J. B.; Mavel, S.; Neyts, J.; Leyssen, P.; Paeshuyse, J.; Gueiffier, A. Eur. J. Med. Chem. 2013, 64, 448.
[13] Patel, H. S.; Linn, J. A.; Drewry, D. H.; Hillesheim, D. A.; Zuercher, W. J.; Hoekstra, W. J. Tetrahedron Lett. 2003, 44, 4077.
[14] Liu, G. P.; Cong, X. F.; He, J. H.; Luo, S. Z.; Wu, D.; Lan, J. B. J. Chem. Res. 2012, 36, 687.
[15] Wu, Z. Q.; Pan, Y. Y.; Zhou, X. G. Synthesis 2011, 2255.
[16] Dixon, L. I.; Carroll, M. A.; Gregson, T. J.; Ellames, G. J.; Harrington, R. W.; Clegg, W. Org. Biomol. Chem. 2013, 11, 5877.
[17] Collins, M. R.; Huang, Q.; Ornelas, M. A.; Scales, S. A. Tetrahedron Lett. 2010, 51, 3528.
[18] Monir, K.; Bagdi, A. K.; Ghosh, M.; Hajra, A. Org. Lett. 2014, 16, 4630.
[19] Enguehard, C.; Renou, J. L.; Collot, V.; Hervet, M.; Rault, S.; Gueiffier, A. J. Org. Chem. 2000, 65, 6572.
[20] Marhadour, S.; Bazin, M. A.; Marchand, P. Tetrahedron Lett. 2012, 53, 297.
[21] Nandi, D.; Jhou, Y. M.; Lee, J. Y.; Kuo, B. C.; Liu, C. Y.; Huang, P. W.; Lee, H. M. J. Org. Chem. 2012, 77, 9384.
[22] Karale, U. B.; Kalari, S.; Shivakumar, J.; Makane, V. B.; Babar, D. A.; Thakare, R. P.; Nagendra Babu, B.; Chopra, S.; Rode, H. B. RSC Adv. 2016, 6, 65095.
[23] Mu, B.; Wu, Y. S.; Li, J. Y.; Zou, D. P.; Chang, J. B.; Wu, Y. J. Org. Biomol. Chem. 2016, 14, 246.
[24] Lee, J.; Chung, J.; Byun, S. M.; Kim, B. M.; Lee, C. Tetrahedron 2013, 69, 5660.
[25] Fu, H. Y.; Chen, L.; Doucet, H. J. Org. Chem. 2012, 77, 4473.
[26] Nandi, D.; Siwal, S. S.; Mallick, K. ChemistrySelect 2017, 2, 1747.
[27] Cao, H.; Zhan, H. Y.; Lin, Y. G.; Lin, X. L.; Du, Z. D.; Jiang, H. F. Org. Lett. 2012, 14, 1688.
[28] Kalari, S.; Babar, D. A.; Karale, U. B.; Makane, V. B.; Rode, H. B. Tetrahedron Lett. 2017, 58, 2818.
[29] Choy, P. Y.; Luk, K. C.; Wu, Y. N.; So, C. M.; Wang, L. L.; Kwong, F. Y. J. Org. Chem. 2015, 80, 1457.
[30] Cao, H.; Lin, Y. G.; Zhan, H. Y.; Du, Z. D.; Lin, X. L.; Liang, Q. M.; Zhang, H. RSC Adv. 2012, 2, 5972.
[31] Liu, Q. X.; He, B. Y.; Qian, P. C.; Shao, L. X. Org. Biomol. Chem. 2017, 15, 1151.
[32] Zhao, G. K.; Zhang, K. N.; Wang, L.; Li, J. Y.; Zou, D. P.; Wu, Y. J.; Wu, Y. S. Tetrahedron Lett. 2015, 56, 6700.
[33] Ke, C. H.; Kuo, B. C.; Nandi, D.; Lee, H. M. Organometallics 2013, 32, 4775.
[34] Frett, B.; McConnell, N.; Smith, C. C.; Wang, Y. X.; Shah, N. P.; Li, H. Y. Eur. J. Med. Chem. 2015, 94, 123.
[35] Hiebel, M. A.; Fall, Y.; Scherrmann, M. C.; Berteina-Raboin, S. Eur. J. Org. Chem. 2014, 2014, 4643.
[36] Cooper, K.; Fray, M. J.; Parry, M. J.; Richardson, K.; Steele, J. J. Med. Chem. 1992, 35, 3115.
/
| 〈 |
|
〉 |