

MCM-41@席夫碱-Mn(OAc)2在水相中催化合成氧杂蒽衍生物
收稿日期: 2017-08-29
修回日期: 2017-11-21
网络出版日期: 2017-12-01
基金资助
国家自然科学基金(Nos.21362036,21161026)资助项目.
MCM-41@Schiff Base-Mn(OAc)2 Catalyzed Synthesis of Xanthene Derivatives in Water
Received date: 2017-08-29
Revised date: 2017-11-21
Online published: 2017-12-01
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21362036, 21161026).
邢刘桩 , 侯亚东 , 邢雪建 , 惠永海 . MCM-41@席夫碱-Mn(OAc)2在水相中催化合成氧杂蒽衍生物[J]. 有机化学, 2018 , 38(4) : 912 -918 . DOI: 10.6023/cjoc201708057
MCM-41@Schiff base-Mn(OAc)2, a heterogeneous catalyst, was used for one pot synthesis of xanthene derivatives in water with aldehydes, 5,5-dimethyl-1,3-cyclohexanedione and 2-naphthol. This method exhibited some advantages including excellent yields, environmentally benign and good functional group tolerance, and the catalytic system presented some features of the less amount of catalyst and excellent reusability by experiment.
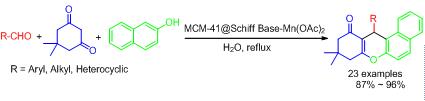
Key words: MCM-41; aqueous phase; xanthene; heterogeneous catalyst
[1] Poupelin, J. P.; Saint-Rut, G.; Fussard-Blanpin, O.; Narcisse, G.; Uchida-Ernouf, G.; Lakroix, R. J. Med. Chem. 1978, 13, 67.
[2] El-Brashy, M. A.; Metwally, M. E.; El-Sepai, F. A. Farmaco 2004, 59, 809.
[3] Madhav, J. V.; Reddy, Y. T.; Reddy, P. N.; Reddy, M. N.; Kuarm, S.; Crooks, P. A.; Rajitha, B. J. Mol. Catal. A 2009, 304, 85.
[4] Adachi, K.; Watanabe, K.; Yamazaki, S. Ind. Eng. Chem. Res. 2014, 53, 13046.
[5] Klimtchuk, E.; Rodgers, M. A. J.; Neckers, D. C. J. Phys. Chem. 1992, 96, 9817.
[6] (a) Niu, G.-L.; Liu, W. M.-B.; Zhou, J.; Xiao, H.-Y.; Wu, J.-S.; Ge, J.-C.; Wang, P.-F. J. Org. Chem. 2016, 81, 7393.
(b) Kamino, S.; Murakami, M.; Tanioka, M.; Shirasaki, Y.; Watanabe, K.; Horigome, J.; Ooyama, Y.; Enomoto, S. Org. Lett. 2014, 16, 258.
(c) Katori, A.; Azuma, E.; Ishimura, H.; Kuramochi, K.; Tsubaki, K. J. Org. Chem. 2015, 80, 4603.
[7] (a) Sun, X.-J.; Zhou, J.-F.; Zhao, P.-S. Synth. Commun. 2012, 42, 1542.
(b) Liang, X.-T.; Ge, D.-L.; Qi, J.-X.; Lu, Y.-H. Acta Pharm. Sin. 1982, 17, 588(in Chinese). (梁晓天, 葛大伦, 祁建新, 卢玉华, 药学学报, 1982, 17, 588.)
[8] (a) Huang, H.; Yao, Y.; Lin, Q.; Zhao, J.; Hua, C.; Gou, X. Russ. J. Gen. Chem. 2016, 86, 934.
(b) Maleki, A.; Aghaei, M.; Ghamari, N. Appl. Organomet. Chem. 2016, 30, 939.
(c) Zhou, B.-D.; Fang, Y.-Y.; Chen, Y.-L.; Lin, S.-F.; Lü, N. J. Putian Univ. 2016, 23, 31(in Chinese). (周北斗, 方圆圆, 陈玉丽, 林淑凤, 吕楠, 莆田学院学报, 2016, 23, 31.)
[9] Tabatabaeian, K.; Khorshidi, A.; Mamaghani, M.; Dadashi, A.; Jalali, M. K. Can. J. Chem. 2011, 89, 623.
[10] L, C.-J. Organic Reactions in Aqueous Media, Ed.:Chan, T. H., Wiley-Interscience, Hoboken, 1997, p. 216.
[11] (a) Luo, F.-H.; Long, Y.; Li, Z.-K.; Zhou, X.-G. Acta Chim. Sinica 2016, 74, 805(in Chinese). (罗飞华, 龙洋, 李正凯, 周向葛, 化学学报, 2016, 74, 805.)
(b) Yang, J.; Fu, T.; Long, Y.; Zhou, X.-G. Chin. J. Org. Chem. 2017, 37, 1111(in Chinese). (杨军, 付婷, 龙洋, 周向葛, 有机化学, 2017, 37, 1111.)
[12] Ostafin, A.; Landefester, K. Boston:Artech House 2009, 1, 78.
[13] (a) An, J.-H.; Cheng, T.-Y.; Xiong, X.; Wu, L.; Han, B.; Liu, G.-H. Catal. Sci. Technol. 2016, 6, 5714.
(b) Chermahini, A. N.; Azadi, M.; Tafakori, E.; Teimouri, A.; Sabzalian, M. J. Porous Mater. 2016, 23, 441.
(c) Wang, S.-S.; He, J.; An, Z. Chem. Commun. 2017, 53, 8882.
[14] (a) Wang, Y.; Liang, M.-X.; Fang, J.-S.; Fu, J.; Chen, X.-C. Chemosphere 2017, 182, 468.
(b) Ding, L.-H.; Li, L.; Chen, Y.; Hong, J.; Wang, L.; Hou, J.; Wang, H.-D. Chin. J. Synth. Chem. 2015, 23, 300(in Chinese). (丁力浩, 李雷, 陈勇, 洪杰, 王亮, 侯杰, 汪海东, 合成化学, 2015, 23, 300.)
[15] (a) Peter, C.; Derible, A.; Parmentier, J.; Drian, C.-L.; Becht, J.-M. New J. Chem. 2017, 41, 4931.
(b) Khanmoradi, M.; Nikoorazm, M. Catal. Lett. 2017, 147, 1114.
[16] Biradar, A.-V.; Patil, V.-S.; Chandra, P.; Doke, D.-S.; Asefa, T. Chem. Commun. 2015, 51, 8496.
[17] Anastas, P. T. Green Chemistry:Theory and Practice, Ed.:Warner, J. C., Oxford University Press, New York, 2000, pp. 19758~19771.
[18] Zhang, Q.; Su, H.; Luo, J.; Wei, Y.-Y. Green Chem. 2012, 14, 201.
[19] Fatma, S.; Singh, P. K.; Ankit, P. Tetrahedron Lett. 2013, 54, 6732.
[20] Shaterian, H. R.; Mohammadnia, M. Res. Chem. Intermed. 2012, 39, 4221.
[21] Taghavi, F.; Gholizadeh, M.; Saljooghi, A. S.; Ramezani, M. RSC Adv. 2016, 6, 87082.
[22] Rama, V.; Kanagaraj, K.; Pitchumani, K. Tetrahedron Lett. 2012, 53, 1018.
[23] Li, J.-J.; Tang, W.-Y.; Lu, L.-M.; Su, W.-K. Tetrahedron Lett. 2008, 49, 7117.
[24] Wang, H.-J.; Ren, X.-Q.; Zhang, Y.-Y.; Zhang, Z.-H. J. Braz. Chem. Soc. 2009, 20, 1939.
[25] Nandi, G. C.; Samai, S.; Kumar, R.; Singh, M. S. Tetrahedron 2009, 65, 7129.
[26] Fu, Y.-H.; Shi, H.-L.; Zhou, G.-P.; Hui, Y.-H.; Xie, Z.-F. Chin. J. Appl. Chem. 2015, 32, 1260(in Chinese). (付亚红, 师红丽, 周广鹏, 惠永海, 解正峰, 应用化学, 2015, 32, 1260.)
/
| 〈 |
|
〉 |