

季铵盐与醛或羧酸的无金属酯化反应研究
收稿日期: 2017-10-26
修回日期: 2017-11-26
网络出版日期: 2017-12-05
基金资助
国家自然科学基金(No.21462031)、内蒙古自治区高等学校青年科技英才支持计划(No.NJYT-17-A22)资助项目.
Metal-Free Esterification of Aldehydes or Carboxylic Acids with Quaternary Ammonium Salts
Received date: 2017-10-26
Revised date: 2017-11-26
Online published: 2017-12-05
Supported by
Project supported by the National Natural Science Foundation of China (No. 21462031) and the Program for Young Talents of Science and Technology in Universities of Inner Mongolia Autonomous Region (No. NJYT-17-A22).
孟根其其格 , 乌云 , 包永胜 . 季铵盐与醛或羧酸的无金属酯化反应研究[J]. 有机化学, 2018 , 38(4) : 902 -911 . DOI: 10.6023/cjoc201710034
A metal-free esterification of various aldehydes or carboxylic acids with quaternary ammonium salts has been developed for selective synthesis of esters. A possible mechanism containing radical process that aldehyde converts to carboxylic acid and the generation of iodohydrocarbon via C-N bond cleavage of quaternary ammonium salt is proposed.
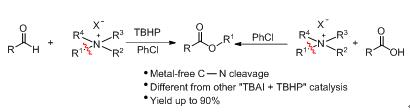
Key words: quaternary ammonium salt; esterification; aldehyde
[1] (a) Cassar, L.; Foa, M.; Gardano, A. J. Organomet. Chem. 1976, 121, C55.
(b) Bhardwaj, M.; Sahi, S.; Mahajan, H.; Paul, S.; Clark, J. H. J. Mol. Catal. A:Chem. 2015, 408, 48.
(c) Zhang, J. T.; Li, D. Y.; Chen, H.; Wang, B. J.; Liu, Z. X.; Zhang, Y. H. Adv. Synth. Catal. 2016, 358, 792.
[2] (a) Wang, X.; Zhu, L. Z.; Chen, S. H.; Xu, X. H.; Au, C. T.; Qiu, R. Org. Lett. 2015, 17, 5228.
(b) Lin, C. L.; Li, D.; Wang, Y. B.; Yao, J. Z.; Zhang, Y. H. Org. Lett. 2015, 17, 1328.
(c) Fabrizi, G.; Goggiamani, A.; Sferrazza, A.; Cacchi, S. Angew. Chem., Int. Ed. 2010, 49, 4067.
(d) Thirupathi, N.; Puri, S.; Reddy, T. J.; Sridhar, B.; Reddya, M. S. Adv. Synth. Catal. 2016, 358, 303.
(e) Xu, P.; Han, F. S.; Wang, Y. H. Adv. Synth. Catal. 2015, 357, 3441.
(f) Gadge, S. T.; Khedkar, M. V.; Lanke, S. R.; Bhanage, B. M. Adv. Synth. Catal. 2012, 354, 2049.
[3] (a) Hirao, T.; Yamada, N.; Ohshiro, Y.; Agawa, T. J. Organomet. Chem. 1982, 236, 409.
(b) Hosomi, A.; Hoashi, K.; Kohra, S.; Tominaga, Y.; Otaka, K.; Sakurai, H. J. Chem. Soc., Chem. Commun. 1987, 570.
(c) Bao, H.; Qi, X.; Tambar, U. K. J. Am. Chem. Soc. 2011, 133, 1206.
[4] (a) Wenkert, E.; Han, A.-L.; Jenny, C.-J. J. Chem. Soc., Chem. Commun. 1988, 975.
(b) Reeves, J. T.; Fandrick, D. R.; Tan, Z.; Song, J. J.; Lee, H.; Yee, N. K.; Senanayake, C. H. Org. Lett. 2010, 12, 4388.
(c) Guo, W.-J.; Wang, Z.-X. Tetrahedron 2013, 69, 9580.
[5] (a) Blakey, S. B.; MacMillan, D. W. C. J. Am. Chem. Soc. 2003, 125, 6046.
(b) Maity, P.; Shacklady-McAtee, D. M.; Yap, G. P. A.; Sirianni, E. R.; Watson, M. P. J. Am. Chem. Soc. 2013, 135, 280.
[6] (a) Xie, L.-G.; Wang, Z.-X. Angew. Chem., Int. Ed. 2011, 50, 4901.
(b) Zhang, X.-Q.; Wang, Z.-X. J. Org. Chem. 2012, 77, 3658.
[7] Zhang, X. Q.; Wang, Z.-X. Org. Biomol. Chem. 2014, 12, 1448.
[8] (a) Uyanik, M.; Ishihara, K. ChemCatChem 2012, 4, 177.
(b) Xue, Q. C.; Xie, J.; Li, H. M.; Cheng, Y. X.; Zhu, C. J. Chem. Commun. 2013, 49, 3700.
(c) Zhao, D.; Wang, T.; Shen, Q.; Li, J. X. Chem. Commun. 2014, 50, 4302.
(d) Wei, W.; Zhang, C.; Xu, Y.; Wan, X. B. Chem. Commun. 2011, 47, 10827.
(e) Liu, L. H.; Yun, L.; Wang, Z. K.; Fu, X. F.; Yan, C. H. Tetrahedron Lett. 2013, 54, 5383.
(f) Li, D. J.; Yang, T. H.; Su, H. L.; Yu, W. Adv. Synth. Catal. 2015, 357, 2529.
(g) Hao, W. J.; Du, Y.; Wang, D.; Jiang, B.; Gao, Q.; Tu, S. J.; Li, G. G. Org. Lett. 2016, 18, 1884.
(h) Zhang, H.; Dong, D. Q.; Hao, S. H.; Wang, Z. L. RSC Adv. 2016, 6, 8465.
(i) Sun, J. W.; Wang, Y.; Pan, Y. J. Org. Chem. 2015, 80, 8945.
(j) Uyanik, M.; Okamoto, H.; Yasui, T.; Ishihara, K. Science 2010, 328, 1376.
(k) Kim, H. J.; Kim, J.; Cho, S. H.; Chang, S. J. Am. Chem. Soc. 2011, 133, 16382.
(l) Rao, H.; Ma, X.; Liu, Q.; Li, Z.; Cao, S.; Li, C.-J. Adv. Synth. Catal. 2013, 355, 2191.
[9] Wei, W.; Zhang, C.; Xu, Y.; Wan, X. Chem. Commun. 2011, 47, 10827.
[10] Liu, Z.; Zhang, J.; Chen, S.; Shi, E.; Xu, Y.; Wan, X. Angew. Chem., Int. Ed. 2012, 51, 3231.
[11] Zhao, J.; Li, P.; Xia, C.; Li, F. Chem. Commun. 2014, 50, 4751.
[12] Deshidi, R.; Rizvi, M. A.; Shah, B. A. RSC Adv. 2015, 5, 90521.
[13] Tan, B.; Toda, N.; Barbas, C. F. Angew. Chem., Int. Ed. 2012, 51, 12538.
[14] Huang, J.; Li, L.; Li, H.; Husan, E.; Wang, P.; Wang, B. Chem. Commun. 2012, 48, 10204.
[15] (a) Fox, S. C.; Li, B.; Xu, D.; Edgar, K. J. Biomacromolecules 2011, 12, 1956.
(b) Otera, J.; Nishikido, J. Esterification, Methods, Reactions, and Applications, 2nd ed., Wiley-VCH, Weinheim, 2010.
(c) Otera, J. Chem. Rev. 1993, 93, 1449.
(d) Swamy, K. C. K.; Kumar, N. N. B.; Balaraman, E.; Kumar, K. V. P. P. Chem. Rev. 2009, 109, 2551.
[16] Kouichi, M.; Hayato, S.; Yu, M.; Kazuaki, S.; Fumi, H.; Yuki, Y.; Hirotsugu, M.; Keiji, N.; Shigenori, K. J. Oleo Sci. 2014, 63, 539.
[17] Kong, W.; Li, B.; Xu, X.; Song, Q. J. Org. Chem. 2016, 81, 8436.
[18] Wang, Q.; Wang, Z.; Xu, Y.; Zhang, X.; Fan, X. Asian J. Org. Chem. 2016, 5, 1304.
[19] Nulty, J.; Nair, J. J.; Sliwinski, M.; Robertson, A. J. Tetrahedron Lett. 2009, 50, 2342.
[20] Blanc, P. Y.; Perret, A.; Teppa, F. Helv. Chim. Acta 1964, 47, 567.
[21] Nishii, Y.; Akiyama, S.; Kita, Y.; Mashima, K. Synlett 2015, 26, 1831.
[22] Tahmasbi, B.; Ghorbani-Choghamarani, A. Catal. Lett. 2017, 147, 649.
[23] Cho, C. S.; Kim, D. T.; Choi, H.; Kim, T.; Shim, S. C. Bull. Korean Chem. Soc. 2002, 23, 539.
[24] Lu, B.; Zhu, F.; Sun, H.; Shen, Q. Org. Lett. 2017, 19, 1132.
[25] Gaspa, S.; Porcheddu, A.; De Luca, L. Org. Lett. 2015, 17, 3666.
[26] Subrao, M.; Potukuchi, D. M.; Ramachandra, G.; Bhagavath, P.; Bhat, S. G.; Maddasani, S. Beilstein J. Org. Chem. 2015, 11, 233.
[27] Liu, H.; Eisen, M. S. Organometallics 2017, 36, 1461.
[28] Beller, M.; Magerlein, W.; Indolese, A. F.; Fischer, C. Synthesis 2001, 1098.
[29] Zhu, Z.; Yuan, J.; Zhou, Y.; Qin, Y.; Xu, J.; Peng, Y. Eur. J. Org. Chem. 2014, 2014, 511.
[30] Bhandari, K. S.; Snieckus, V. Can. J. Chem. 1971, 49, 2354.
/
| 〈 |
|
〉 |