

三氟甲基亚磺酸钠实现的三氟甲基化反应研究进展
收稿日期: 2017-09-08
修回日期: 2017-11-09
网络出版日期: 2017-12-08
基金资助
江苏省自然科学基金(No.BK20140136)及江苏高校品牌专业建设工程(No.PPZY2015B146)资助项目.
Research Progress of Trifluoromethylation with Sodium Trifluoromethanesulfinate
Received date: 2017-09-08
Revised date: 2017-11-09
Online published: 2017-12-08
Supported by
Project supported by the Natural Science Foundation of Jiangsu Province (No. BK20140136) and the Top-notch Academic Programs Project of Jiangsu Higher Education Institutions (No. PPZY2015B146).
惠人杰 , 张士伟 , 谭政 , 吴小培 , 冯柏年 . 三氟甲基亚磺酸钠实现的三氟甲基化反应研究进展[J]. 有机化学, 2017 , 37(12) : 3060 -3075 . DOI: 10.6023/cjoc201709011
Trifluoromethyl can increase the chemical and metabolic stability of drugs, improve its lipophilicity and bioavailability, and furthermore, enhance drug binding selectivities. Sodium trifluoromethanesulfinate (CF3SO2Na) is a stable inexpensive reagent, which has been widely used in the field of organic fluorine chemistry. The recent progress (2014~2017) in trifluoromethylation by employing CF3SO2Na as the trifluoromethyl source is summarized. In addition, the reactions of bifunctionalization, trifluoromethylation of aromatics, trifluoromethylthioization and other types of reactions are described respectively, with their applications and reaction mechanism. It is hoped that this review can be referred to in the studies of trifluoromethyl introduction.
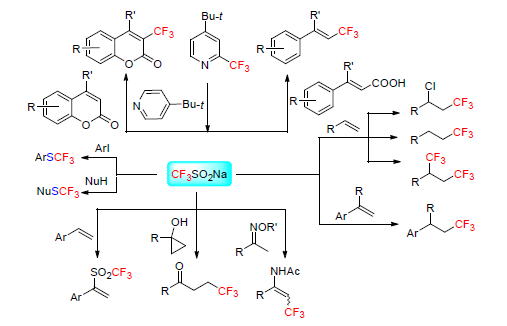
[1] Grem J. L. Invest. New Drugs 2000, 18, 299
[2] Krik, K. L. Org. Process Res. Dev. 2008, 12, 305
[3] Yang, B.; Xu, X. H.; Qing, F. L. Org. Lett. 2015, 17, 1906.
[4] Liu, X.; Xu, C.; Wang, M. Chem. Rev. 2015, 115, 683.
[5] Yang, F.; Klumphu, P.; Liang, Y. M. Chem. Commun. 2014, 50, 936.
[6] Ji, Y.; Brueckl, T.; Baxter, R. D.; Fujiwara, Y.; Seiple, I. B.; Su, S.; Blackmond, D. G.; Baran, P. S. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 14411.
[7] Umemoto, T. Chem. Rev. 1996, 96, 1757.
[8] Eisenberger, P.; Gischig, S.; Togni, A. Chemistry 2006, 12, 2579.
[9] Ryota, H.; Toshiaki, I.; Kohsuke, A. Chemistry 2014, 20, 2750.
[10] Morimoto, H.; Tsubogo, T.; Litvinas, N. D. Angew Chem., Int. Ed. 2011, 50, 3793.
[11] Tomashenko, O. A.; Escudero, A. E. C.; Martínez, B. M. Angew Chem., Int. Ed. 2011, 50, 7655.
[12] Zheng, J.; Lin, J. H.; Deng, X. Y. Org. Lett. 2015, 17, 532.
[13] Maji, A.; Hazra, A.; Maiti, D. Org. Lett. 2014, 16, 4524.
[14] (a) Lefebvre, Q. Synlett 2016, 28, 19.
(b) Tordeux, M.; Langlois, B. R.; Wakselman, C. J. Org. Chem. 1989, 54, 2452.
[15] Zhang, C. Adv. Synth. Catal. 2014, 356, 2895.
[16] Lu, Y.; Li, Y.; Zhang, R. J. Fluorine Chem. 2014, 161, 128.
[17] Hang, Z.; Li, Z.; Liu, Z. Q. Org. Lett. 2014, 16, 3648.
[18] Zhu, L.; Wang, L. S.; Li, B. Chem. Commun. 2016, 52, 6371.
[19] Yang, B.; Xu, X. H.; Qing, F. L. Chin. J. Chem. 2016, 34, 465.
[20] Yang, Y.; Liu, Y.; Jiang, Y.; Zhang, Y.; Vicic, D. A. J. Org. Chem. 2015, 80, 6639.
[21] Yang, B.; Xu, X. H.; Qing, F. L. Org. Lett. 2015, 17, 1906.
[22] Liu, X.; Xiong, F.; Huang, X.; Xu, L.; Li, P.; Wu, X. Angew. Chem., Int. Ed. 2013, 52, 6962.
[23] Chen, Z. M.; Bai, W.; Wang, S. H.; Yang, B. M.; Tu, Y. Q.; Zhang, F. M. Angew. Chem., Int. Ed. 2013, 52, 9781.
[24] Egami, X.; Shimizu, R.; Usuiac, Y.; Sodeoka, M. Chem. Commun. 2013, 49, 7346.
[25] Huang, H. L.; Yan, H.; Gao, G. L. Asian J. Org. Chem. 2015, 4, 674.
[26] Lu, Q.; Liu, C.; Huang, Z. Chem. Commun. 2014, 50, 14101.
[27] Liu, C.; Lu, Q.; Huang, Z. Org. Lett. 2015, 17, 6034.
[28] Li, B.; Fan, D.; Yang C; Xia W. Org. Biomol. Chem. 2016, 14, 5293.
[29] Yu, J.; Yang, H.; Fu, H. Adv. Synth. Catal. 2014, 356, 3669.
[30] Mu, X.; Wu, T.; Wang, H. Y.; Guo, Y. L.; Liu, G. S. J. Am. Chem. Soc. 2012, 134, 878.
[31] Wei, W.; Wen, J.; Yang, D. J. Org. Chem. 2014, 79, 4225.
[32] Hua, H. L.; He, Y. T.; Qiu, Y. F. Chem.-Eur. J. 2015, 21, 1468.
[33] Zhang, L. Z.; Li, Z. J.; Liu, Z. Q. Cheminform 2014, 46, 3648.
[34] Jana, S.; Verma, A.; Kadu, R.; Kumar, S. Chem. Sci. 2017, 8, 6633.
[35] Wu, L. H.; Zhao, K.; Shen, Z. L.; Loh, T. P. Org. Chem. Front. 2017, 4, 1872.
[36] Martin, R.; Reddy, Y. V.; Shen, Y. Angew. Chem., Int. Ed. 2017, 56, 10915.
[37] Zhang, X.; Huang, P.; Li, Y. Org. Biomol. Chem. 2015, 13, 10917.
[38] Zhang, K.; Xu, X. H.; Qing, F. L. J. Org. Chem. 2015, 80, 7658.
[39] Wu, M.; Ji, X.; Dai, W. J. Org. Chem. 2014, 79, 8984.
[40] (a) Cai, S. J.; Chen, C.; Sun, Z. L.; Xi, C. J. Chem. Commun. 2013, 49, 4552.
(b) Zhang, L. S.; Chen, K.; Chen, G. H.; Li, B. J.; Luo, S.; Guo, Q. Y.; Wei, J. B.; Shi, Z. J. Org. Lett. 2013, 15, 10.
[41] Ji, X. M.; Wei, L.; Chen, F. RSC Adv. 2015, 46, 29766.
[42] (a) Cho, E. J.; Senecal, T. D.; Kinzel, T. Y.; Zhang, Y.; Watson, D. A.; Buchwald, S. L. Science 2010, 328, 1679.
(b) Oishi, M.; Kondo H.; Amii, H. Chem. Commun. 2009, 1909.
(c) Chu, L.; Qing, F. L. Org. Lett. 2010, 12, 5060.
(d) Senecal, T. D.; Parsons, A. T.; Buchwald, S. L. J. Org. Chem. 2011, 76, 1174.
(e) Jiang, X.; Chu, L.; Qing, F. L. J. Org. Chem. 2012, 77, 1251.
(f) Herrmann, A. T.; Smith, L. L.; Zakarian, A. J. Am. Chem. Soc. 2012, 134, 6976.
(g) Sato, K.; Omote, M.; Ando, A.; Kmadaki, I. Org. Lett. 2004, 6, 4359.
(h) Shimizu, R.; Egami, H.; Nagi, T.; Chae, J.; Hamashima, Y.; Sodeoka, M. Tetrahedron Lett. 2010, 51, 5947.
(i) Liu, T.; Shen, Q. Org. Lett. 2011, 13, 2342.
(j) Nagib, D. A. M.; MacMillan, D. W. C. Nature 2011, 480, 224.
[43] Fennewald, J. C.; Lipshutz, B. H. Green Chem. 2014, 16, 1097.
[44] Cao, X.; Pan, X.; Zhou, P. Chem. Commun. 2014, 50, 3359.
[45] Lu, Y.; Li, Y.; Zhang, R. J. Fluorine Chem. 2014, 161, 128.
[46] Pair, E.; Monteiro, N.; Bouyssi, D.; Baudoin, O. Angew. Chem., Int. Ed. 2013, 52, 5346.
[47] Tan, Z.; Zhang, S. W.; Zhang, Y. J. Org. Chem. 2017, 82, 9384.
[48] Jiang, H. F.; Huang, W.; Yu, Y.; Yi, S. J.; Li, J. W.; Wu, W. Q. Chem. Commun. 2017, 53, 7473.
[49] Li, C. F.; Suzuki, K.; Yamaguchi, K.; Mizuno, N. New J. Chem. 2017, 41, 1471.
[50] Simon, R. C.; Busto, E.; Richter, N.; Resch, V.; Houk, K. N.; Kroutil W. Nat. Commun. 2016, 7, 13323.
[51] Bu, M.; Lu, G.; Cai, C. T. Org. Chem. Front. 2017, 4, 266.
[52] Yang, Y.; Xu, L.; Yu, S. Chem.-Eur. J. 2016, 22, 858.
[53] Zhao, X.; Wei, A. Q.; Yang, B.; Li, T. J.; Li, Q.; Qiu, D.; Lu, K. J. Org. Chem. 2017, 82, 9175.
[54] Jiang, L.; Qian, J.; Yi, W.; Lu, G.; Cai, C.; Zhang, W. Angew. Chem., Int. Ed. 2015, 54, 14965.
[55] Yang, Y.; Xu, L.; Yu, S.; Liu, X.; Zhang, Y.; Vicic, D. A. Chem.-Eur. J. 2016, 22, 858.
[56] Chachignon, H.; Maeno, M.; Kondo, H.; Shibata, N.; Cahard, D. Org. Lett. 2016, 18, 2467.
[57] Hua, L. N.; Li, H.; Qing, F. L. Org. Biomol. Chem. 2016, 14, 8443.
[58] (a) Shangary, S.; Johnson, D. E. Leukemia 2003, 17, 1470.
(b) Oltersdorf, T.; Elmore, S. W.; Shoemaker, A. R.; Armstrong, R. C.; Augeri, D. J.; Belli, B. A.; Bruncko, M.; Deckwerth, T. L.; Dinges, J.; Hajduk, P. J.; Joseph, M. K.; Kitada, S.; Korsmeyer, S. J.; Kunzer, A. R.; Letai, A.; Li, C.; Mitten, M. J.; Nettesheim, D. G.; Ng, S.; Nimmer, P. M.; O'Connor, J. M.; Oleksijew, A.; Petros, A. M.; Reed, J. C.; Shen, W.; Tahir, S. K.; Thompson, C. B.; Tomaselli, K. J.; Wang, B.; Wendt, M. D.; Zhang, H.; Fesik, S. W.; Rosenberg, S. H. Nature 2005, 435, 677;
(c) Morizawa, Y.; Okazoe, T.; Wang, S. Z.; Sasaki, J.; Ebisu, H.; Nishikawa, M.; Shinyama, H. J. J. Fluorine Chem. 2001, 109, 83.
[59] Smyth, L. A. J. Org. Chem. 2016, 81, 1285.
[60] Konik, Y. A.; Kudrjashova, M.; Konrad N.; Kaabel, S.; Järving, I.; Lopp, M.; Kananovich, D. G. Org. Biomol. Chem. 2017, 15, 4635.
[61] van der Werf, A.; Hribersek, M.; Selander, N. Org. Lett. 2017, 19, 2374.
[62] Yang, H. B.; Selander, N. Org. Biomol. Chem. 2017, 15, 1771.
[63] Han, J. B.; Yang, L.; Chen, X.; Zha, G. F.; Zhang, C. P. Adv. Synth. Catal. 2016, 358, 4119.
[64] Liao, Y. Y.; Deng, J. C.; Ke, Y. P.; Zhong, X. L.; Xu, L.; Tang, R. Y.; Zheng, W. Chem. Commun. 2017, 53, 6073.
/
| 〈 |
|
〉 |