

基于天然产物Mevalocidin手性中心的结构类似物的设计与合成
收稿日期: 2017-10-21
修回日期: 2017-11-24
网络出版日期: 2017-12-15
基金资助
国家自然科学基金(No.21472063)资助项目.
Design and Synthesis of Natural Product Mevalocidin Chiral Center Based Analogues
Received date: 2017-10-21
Revised date: 2017-11-24
Online published: 2017-12-15
Supported by
Project supported by the National Natural Science Foundation of China (No. 21472063).
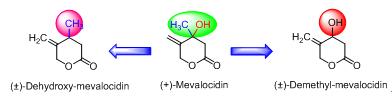
Mevalocidin是从Rosellinia DA092917和Fusarium DA056446菌株中分离到的一种天然产物,在4 kg/ha的高剂量下具有苗后除草活性.希望对其手性中心进行改造以提高除草活性,通过设计不同合成路线,合成了2个类似物,通过1H NMR、13C NMR及HRMS对所合成化合物的结构进行了确认.活性筛选结果表明,所合成的目标化合物不具有除草活性,说明天然产物手性中心上的甲基和羟基对其活性保持至关重要,为进一步结构修饰提供了指导.
关键词: 天然产物; Mevalocidin; 结构改造; 手性中心; 除草活性
吴琼友 , 张锐 , 潘金环 , John Clough , 顾玉成 , 杨光富 . 基于天然产物Mevalocidin手性中心的结构类似物的设计与合成[J]. 有机化学, 2018 , 38(4) : 840 -845 . DOI: 10.6023/cjoc201710023
Mevalocidin is a phytotoxin, produced by two fungal isolates designated Rosellinia DA092917 and Fusarium DA056446, and has a broad spectrum of post emergence herbicidal activity at relatively high rate (4 kg/ha). Structural modification of mevalocidin at its chiral center was investigated with the aim of discovering of novel herbicides with improved activity. In order to determine the role of hydroxyl and methyl groups of mevalocidin exhibiting the herbicidal activity, we designed (±)-deoxy-mevalocidin (1) and (±)-demethyl-mevalocidin (2), respectively. These two designed compounds were synthesized (racemate) through two different synthetic approaches and characterized by 1H NMR, 13C NMR and HRMS spectra. The herbicidal activities of the synthesized compounds were evaluated against both dicotyledon and monocotyledon weeds. Bioassay results indicated that the both synthesized analogues lost the herbicidal activity when compared to parent and it demonstrated that methyl and/or hydroxyl groups at the chiral center of mevalocidin are crucial for its herbicidal activity. Thus, this study will provide insights into the structural modification of mevalocidin.

[1] Guan, A. Y.; Liu, C. L.; Yang, X. P.; Dekeyser, M. Chem. Rev. 2014, 114, 7079.
[2] Hashidoko, Y.; Shinano, T. J. Pest. Sci. 2011, 36, 106.
[3] Jeschke, P. Pest Manage. Sci. 2010, 66, 10.
[4] Damalas, C. A.; Eleftherohorinos, I. G. Int. J. Environ. Res. Public Health 2011, 8, 1402.
[5] Duke, S. O. Pest Manage. Sci. 2012, 68, 505.
[6] Dayan, F. E.; Owens, D. K.; Duke, S. O. Pest Manage. Sci. 2012, 68, 519.
[7] Butler, M. S. J. Nat. Prod. 2004, 67, 2141.
[8] Dayan, F. E.; Duke, S. O. Plant Physiol. 2014, 166, 1090.
[9] Zhang, Z.; Xing, A.; Staswick, P. Clemente, T. E. Plant Cell, Tissue Organ Cul. 1999, 56, 37.
[10] Bayer, E.; Gugel, K. H.; Haegele, K.; Hagenmaier, H.; Esipov, S. E.; Koenig, W. A.; Zaehner, H. Helv. Chim. Acta 1972, 55, 224.
[11] Omura, S.; Hinotozawa, K.; Imamura, N.; Murata, M. J. Antibiot. 1984, 37, 939.
[12] Hoerlein, G. Rev. Environ. Contam. Toxicol. 1994, 138, 73.
[13] Mitchell, G.; Bartlett, D. W.; Fraser, T. E. M.; Hawkes, T. R.; Holt, D. C.; Townson, J. K.; Wichert, R. A. Pest Manage. Sci. 2001, 57, 120.
[14] Gerwick, B. C.; Graupner, P. R.; Fields, S. C.; Schmitzer, P. R.; Brewster, W. K. US 7393812, 2008[Chem. Abstr. 2006, 485213].
[15] Lichtner, F. Austral. J. Plant Physiol. 2000, 27, 609.
[16] Gerwick, B. C.; Brewser, W. K.; de Bore, G. J.; Fields, S. C.; Graupner, P. R.; Hahn, D. R.; Pearce, C. J.; Schmitzer, P. R.; Webster, J. D. J. Chem. Ecol. 2013, 39, 253.
[17] Lin, L.; Mulholland, N.; Wu, Q. Y.; Beattie, D.; Huang, S. W.; Irwin, D.; Clough, J.; Gu, Y. C.; Yang, G. F. J. Agric. Food Chem. 2012, 60, 4480.
[18] Lin, L.; Mulholland, N.; Huang, S. W.; Beattie, D.; Irwin, D.; Gu, Y. C.; Clough, J.; Wu, Q. Y.; Yang, G. F. Chem. Biol. Drug Des. 2012, 80, 682.
[19] Larsen, C. H.; Ridgway, B. H.; Shaw, J. T.; Woerpel, K. A. J. Am. Chem. Soc. 1999, 121, 12208.
[20] Villieras, J.; Rambaud, M. Synth. Commun. 1983, 300.
[21] Suizu, H.; Shigeoka, D.; Aoyama, H.; Yoshimitsu, T. Org. Lett. 2015, 17, 126.
/
| 〈 |
|
〉 |