

新型1,3,5-三嗪-1H-吡唑-三唑并噻二唑衍生物的合成及活性评价
收稿日期: 2017-04-17
修回日期: 2017-06-28
网络出版日期: 2018-01-03
基金资助
辽宁省教育厅科学技术研究(No.2009A426)资助项目.
Synthesis and Bioactivity Evaluation of Novel 1, 3, 5-Triazine-1H-pyrazole-triazol-ethiadiazole Derivatives
Received date: 2017-04-17
Revised date: 2017-06-28
Online published: 2018-01-03
Supported by
Project supported by the Science and Technology Research Program of Liaoning Provincial Department of Education (No. 2009A426).
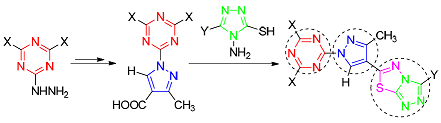
以具有优秀药理活性的吡唑杂环为核心,连接1,3,5-三嗪杂环,引入三唑并噻二唑稠并环,设计合成了21个新型1,3,5-三嗪-1H-吡唑-三唑并噻二唑衍生物.应用IR,1H NMR和HRMS等对21种新物质进行了结构表征.评价了21种新型目标产物对Cdc25B和PTP1B的抑制活性,结果发现,大部分目标分子显示了优良的抑制活性.在Cdc25B抑制活性测试中,14个目标分子抑制活性高于阳性参照物Na3VO4,有望成为潜在的Cdc25B抑制剂,在PTP1B抑制活性测试中,9个目标分子抑制活性优于阳性参照物齐墩果酸,有望成为潜在的PTP1B抑制剂.
张成路 , 李传银 , 顾耀东 , 孙晓娜 , 唐杰 , 王静 , 李益政 , 王华玉 . 新型1,3,5-三嗪-1H-吡唑-三唑并噻二唑衍生物的合成及活性评价[J]. 有机化学, 2018 , 38(5) : 1223 -1232 . DOI: 10.6023/cjoc201704028
Twenty-one novel 1, 3, 5-triazine-1H-pyrazole-triazolethiadiazole derivatives are designed and synthesized, in which the excellent pharmacological activitive pyrazole is used as key skeleton by connecting 1, 3, 5-triazine and introducing triazolothiadiazole. The structures of 21 target compounds are characterized by IR, 1H NMR and HRMS. The inhibitory activities of 21 novel target products against Cdc25B and PTP1B are evaluated. As a result, most of the target compounds exhibit excellent inhibitory activities. In the Cdc25B inhibitory activity test, 14 target compounds have higher inhibitory activities than the positive reference sodium orthovanadate, and they are expected to be potential of Cdc25B inhibitors. In the PTP1B inhibitory activity test, 9 target compounds have better inhibitory activities than the control oleanolic acid, and they are expected to be potential of PTP1B inhibitors.

Key words: pyrazole; 1, 3, 5-triazine; triazolothiadiazole; Cdc25B inhibitor; PTP1B inhibitor
[1] Salahuddin; Shaharyar, M.; Mazumder, A.; Ahsan, M. J. Arabian J. Chem. 2014, 7, 418.
[2] Yang, L.; Yan, J.-F.; Fan, L.; Chen, X.; Shangguan, R.-Y.; Wang, L.-F.; Yang, D.-C. Chin. J. Org. Chem. 2012, 32, 1908(in Chinese). (杨龙, 晏菊芳, 范莉, 陈欣, 上官瑞燕, 汪林发, 杨大成, 有机化学, 2012, 32, 1908.)
[3] (a) Neel, B. G.; Tonks, N. K. Curr. Opin. Cell Biol. 1997, 9, 193.
(b) Hunter, T. Cell 2000, 100, 113.
(c) Tonks, N. K.; Neel, B. G. Curr. Opin. Cell Biol. 2001, 13, 182.
[4] Lavecchia, A.; Giovanni, C. D.; Pesapane, A.; Montuori, N.; Ragno, P.; Martucci, N. M.; Masullo, M.; Vendittis, E. D.; Novellino, E. J. Med. Chem. 2012, 55, 4142.
[5] Zhang, Z.-Y. Annu. Rev. Pharmacol. Toxicol. 2002, 42, 209.
[6] Maccari, R.; Paoli, P.; Ottanà, R.; Jacomelli, M.; Ciurleo, R.; Manao, G.; Steindl, T.; Langer, T.; Vigorita, M. G.; Camici, G. Bioorg. Med. Chem. 2007, 15, 5137.
[7] Li, Y.; Yu, Y.; Jin, K.; Gao, L.; Luo, T.; Sheng, L.; Shao, X.; Li, J. Bioorg. Med. Chem. Lett. 2014, 24, 4125.
[8] Owen, C.; Czopek, A.; Agouni, A.; Grant, L.; Judson, R.; Lees, E. K.; McIlroy, G. D.; Goransson, O.; Welch, A.; Bence, K. K.; Kahn, B. B.; Neel, B. G.; Mody, N.; Delibegovic, M. PloS One 2012, 7, 32693.
[9] Farag, A. M.; Mayhoub, A. S.; Barakat, S. E.; Bayomi, A. H. Bioorg. Med. Chem. 2008, 16, 881.
[10] Xia, Y.; Fan, C.-D.; Zhao, B.-X.; Zhao, J.; Shin, D.-S.; Miao, J.-Y. Eur. J. Med. Chem. 2008, 43, 2347.
[11] Xia, Y.; Dong, Z.-W.; Zhao, B.-X.; Ge, X.; Meng, N.; Shin, D.-S.; Miao, J.-Y. Bioorg. Med. Chem. 2007, 15, 6893.
[12] Rao, K. S.; Ramesh, P.; Trivedi, R.; Kantam, M. L. Tetrahedron Lett. 2016, 57, 1227.
[13] Silvestri, R.; Cascio, M. G.; Regina, G. L.; Piscitelli, F.; Lavecchia, A.; Brizzi, A.; Pasquini, S.; Botta, M.; Novellino, E.; Marzo, V. D.; Corelli, F. J. Med. Chem. 2008, 51, 1560.
[14] Wittman, M. D.; Carboni, J. M.; Yang, Z.; Lee, F. Y.; Antman, M.; Attar, R.; Balimane, P.; Chang, C.; Chen, C.; Discenza, L.; Frennesson, D.; Gottardis, M. M.; Greer, A.; Hurlburt, W.; Johnson, W.; Langley, D. R.; Li, A.; Li, J.; Liu, P.; Mastalerz, H.; Mathur, A.; Menard, K.; Patel, K.; Sack, J.; Sang, X.; Saulnier, M.; Smith, D.; Stefanski, K.; Trainor, G.; Velaparthi, U.; Zhang, G.; Zimmermann, K.; Vyas, D. M. J. Med. Chem. 2009, 52, 7360.
[15] (a) Kuo, G.-H.; DeAngelis, A.; Emanuel, S.; Wang, A.-H.; Zhang, Y.; Connolly, P. J.; Chen, X.; Gruninger, R. H.; Rugg, C.; Fuentes-Pesquera, A.; Middleton, S. A.; Jolliffe, L.; Murray, W. V. J. Med. Chem. 2005, 48, 4535.
(b) Robert, J.; Jarry, C. J. Med. Chem. 2003, 46, 4805.
[16] Misra, R. N.; Xiao, H.-Y.; Williams, D. K.; Kim, K. S.; Lu, S.; Keller, K. A.; Mulheron, J. G.; Batorsky, R.; Tokarski, J. S.; Sack, J. S.; Kimball, S. D.; Lee, F. Y.; Webster, K. R. Bioorg. Med. Chem. Lett. 2004, 14, 2973.
[17] Badr, S. M. I.; Barwa, R. M. Bioorg. Med. Chem. 2011, 19, 4506.
[18] Guzeldemirci, N. U.; Kucukbasmaci, O. Eur. J. Med. Chem. 2010, 45, 63.
[19] Taori, K.; Paul V. J.; Luesch, H. J. Am. Chem. Soc. 2008, 130, 1806.
[20] Pettit, G. R.; Singh, S. B.; Niven, M. L.; Hamel, E.; Schmidt, J. M. J. Nat. Prod. 1987, 50, 119.
[21] Pettit, G. R.; Singh, S. B.; Hamel, E.; Lin, C. M.; Alberts, D. S.; Garcia-Kendall, D. Experientia 1989, 45, 209.
[22] Romagnoli, R.; Baraldi, P. G.; Kimatrai, S. M.; Preti, D.; Aghazadeh, T. M.; Bassetto, M.; Brancale, A.; Hamel, E.; Castagliuolo, I.; Bortolozzi, R.; Basso, G.; Viola, G. J. Med. Chem. 2013, 56, 2606.
[23] Chaudhary, V.; Venghateri, J. B.; Dhaked, H. P.; Bhoyar, A. S.; Guchhait, S. K.; Panda, D. J. Med. Chem. 2016, 59, 3439.
[24] Madadi, N. R.; Penthala, N. R.; Howk, K.; Ketkar, A.; Eoff, R. L.; Borrelli, M. J.; Crooks, P. A. Eur. J. Med. Chem. 2015, 103, 123.
[25] Qin, Y.-J.; Li, Y. J.; Jiang, A.-Q.; Yang, M.-R.; Zhu, Q.-Z.; Dong, H.; Zhu, H. L. Eur. J. Med. Chem. 2015, 94, 447.
[26] Zhang, Q.; Peng, Y.; Wang, X.-I.; Keenan, S. M.; Arora, S.; Welsh, W. J. J. Med. Chem. 2007, 50, 749.
[27] Kamel, M. M.; Megally Abdo, N. Y. Eur. J. Med. Chem. 2014, 86, 75.
[28] Matsuno, T.; Kato, M.; Tsuchida, Y.; Takahashi, M.; Yaguchi, S.; Terada, S. Chem. Pharm. Bull. 1997, 45, 291.
[29] Zhu, W.; Liu, Y.; Zhao, Y.; Wang, H.; Tan, L.; Fan, W.; Gong, P. Arch. Pharm. 2012, 345, 812.
[30] Zhang, C.-L.; Sun, X.-N.; Li, C.-Y.; Cai, J.-Y.; Wang, J.; Li, Y.-Z.; Wang, H.-Y. Chem. J. Chin. Univ. 2017, 38, 1764(in Chinese). (张成路, 孙晓娜, 李传银, 蔡继颖, 王静, 李益政, 王华玉, 高等学校化学学报, 2017, 38, 1764.)
[31] Zhang, C.-L.; Tang, J.; Yin, L.-Y. Xi, H. Guo, Y.; Sun, L.-J. Chin. J. Org. Chem. 2016, 36, 358(in Chinese). (张成路, 唐杰, 殷立莹, 袭焕, 国阳, 孙丽杰, 有机化学, 2016, 36, 358.)
[32] Bijul, L. A.; Gupta, R. L. Indian J. Chem. 2010, 49B, 1235.
[33] Wang, X. M.S. Thesis, Liaoning Normal University, Dalian, 2015 (in Chinese). (王雪, 硕士论文, 辽宁师范大学, 大连, 2015.)
[34] Park, H.; Bahn, Y. J.; Jung, S-K.; Jeong, D.G.; Lee, S-H.; Seo, I.; Yoon, T-S.; Kim, S. J.; Ryu, S. E. J. Med. Chem. 2008, 51, 5533.
[35] Baskaran, S. K.; Goswami, N.; Selvaraj, S.; Muthusamy, V. S.; Lakshmi, B. S. J. Chem. Inf. Model. 2012, 52, 2004.
/
| 〈 |
|
〉 |