

新型酰基硫脲衍生物的合成及细胞分裂周期25B磷酸酶和蛋白酪氨酸磷酸酶1B抑制活性研究
收稿日期: 2017-09-13
修回日期: 2017-12-04
网络出版日期: 2018-01-03
基金资助
辽宁省自然科学基金(No.20102126)资助项目.
Synthesis and Cell Division Cycle 25B Phosphatase/Protein Tyrosine Phosphatase 1B Inhibitory Activity Evaluation of Novel Acylthiourea Derivatives
Received date: 2017-09-13
Revised date: 2017-12-04
Online published: 2018-01-03
Supported by
Project supported by the Natural Science Foundation of Liaoning Province (No. 20102126).
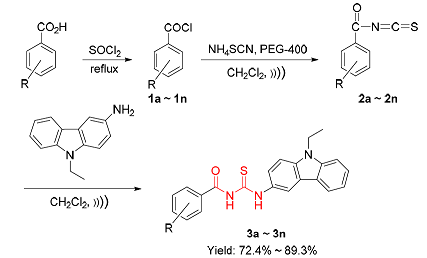
采用超声波辐射与固-液相转移催化联用技术合成出了一系列新型含咔唑基团的酰基硫脲衍生物3,利用IR、1H NMR、13C NMR和元素分析对其进行了结构表征.该合成方法具有反应时间短、操作简便、产率高等优点.对所合成的目标化合物进行了细胞分裂周期25B磷酸酶(Cdc25B)和蛋白酪氨酸磷酸酶1B(PTP1B)抑制活性筛选,实验结果显示,目标化合物3对Cdc25B均具有良好的抑制活性,部分化合物对PTP1B也表现出良好的抑制活性.其中1-(4-硝基苯甲酰基)-3-(9-乙基-咔唑-3-基)硫脲(3n)对Cdc25B的抑制活性最高[IC50=(0.49±0.12)mg/mL],1-(2-硝基苯甲酰基)-3-(9-乙基-咔唑-3-基)硫脲(3l)对PTP1B的抑制活性最高[IC50=(3.59±1.15)mg/mL].值得注意的是,化合物3n对Cdc25B和PTP1B均具有较高的抑制活性.分子对接的初步研究结果揭示了此类抑制剂的结构-活性关系.这些活性目标化合物是潜在的Cdc25B和PTP1B抑制剂,在癌症和糖尿病治疗方面具有很好的应用前景.
关键词: 酰基硫脲; 咔唑; 合成; Cdc25B和PTP1B抑制剂; 分子对接
李英俊 , 王思远 , 靳焜 , 高立信 , 盛丽 , 张楠 , 杨凯栋 , 赵月 , 李佳 . 新型酰基硫脲衍生物的合成及细胞分裂周期25B磷酸酶和蛋白酪氨酸磷酸酶1B抑制活性研究[J]. 有机化学, 2018 , 38(5) : 1242 -1250 . DOI: 10.6023/cjoc201709022
A series of new acylthiourea derivatives 3 containing carbazole moity have been synthesized by the techniques of ultrasonic irradiation and solid-liquid phase transfer catalysis. Their structures were characterized by IR, 1H NMR, 13C NMR spectra and elemental analysis. This synthetic method has the advantages of short reaction time, simple operation and high yield. All synthesized target compounds were screened for their inhibitory activity against cell division cycle 25B phosphatase (Cdc25B) and protein tyrosine phosphatase 1B (PTP1B). The results show that all the compounds 3 display significant inhibitory activities against Cdc25B, and partial target compounds 3 also show significant inhibitory activities against PTP1B. Among them, 1-(4-nitrobenzoyl)-3-(9-ethyl-carbazole-3-yl)thiourea (3n) exhibits highest inhibitory activity against Cdc25B [IC50=(0.49±0.12) mg/mL] and 1-(2-nitrobenzoyl)-3-(9-ethyl-carbazole-3-yl)thiourea (3l) displays highest inhibitory activity against PTP1B [IC50=(3.59±1.15) mg/mL]. It is noteworthy that compound 3n shows higher inhibitory activity against Cdc25B and PTP1B. The preliminary research results of molecular docking revealed the structural-activity of the inhibitors. The active compounds can be considered as potential Cdc25B and PTP1B inhibitors, and have great application prospects in the treatment of cancers and diabetes.

Key words: acylthiourea; carbazole; synthesis; Cdc25B and PTP1B inhibitors; molecular docking
[1] Nitulescu, G. M.; Draghici, C.; Olaru, O. T.; Matei, L.; Ioana, A.; Dragu, L. D.; Bleotu, C. Bioorg. Med. Chem. 2015, 23, 5799.
[2] Jin, L.; Qu, H. E.; Huang, X. C.; Pan, Y. M.; Liang, D.; Chen, Z. F.; Wang, H. S.; Zhang, Y. Int. J. Mol. Sci. 2015, 16, 14571.
[3] Yun, T.; Qin, T.; Liu, Y.; Lai, L. H. Eur. J. Med. Chem. 2016, 124, 229.
[4] Gunasekaran, N.; Vadivel, V.; Halcovitch, N. R.; Tiekink, E. R. T. Chem. Data Collect. 2017, 9, 263.
[5] Kulabas, N.; Özakpinar, Ö. B.; Özsavci, D.; Leyssen, P.; Neyts, J.; Küçükgüzel, I. Marmara Pharm. J. 2017, 21, 371.
[6] Banaei, A.; Shiran, J. A.; Saadat, A.; Ardabili, F. F.; McArdle, P. J. Mol. Struct. 2015, 1099, 427.
[7] Tahir, S.; Badshah, A.; Hussain, R. A.; Tahir, M. N.; Tabassum, S.; Patujo, J. A.; Rauf, M. K. J. Mol. Struct. 2015, 1099, 215.
[8] Cui, P. L.; Li, X. L.; Zhu, M. Y.; Wang, B. H.; Liu, J.; Chen, H. Bioorg. Med. Chem. Lett. 2017, 27, 2234.
[9] Zullkiplee, W. S. H. W.; Ariff, M. A. M.; Hussain, H.; Khairul W. M.; Ngaini, Z. Phosphorus, Sulfur Silicon Relat. Elem. 2016, 191, 1329.
[10] Khairul, W. M.; Ariffin, A. A.; Ismail, N.; Daud, A. I. Educ. Jsmt. 2016, 3, 13.
[11] Halim, A. N. A.; Ngaini, Z. Phosphorus, Sulfur Silicon Relat. Elem. 2017, 192, 1.
[12] Sun, C. W.; Huang, H.; Feng, M. Q.; Shi, X. L.; Zhang, X. D.; Zhou, P. Bioorg. Med. Chem. Lett. 2006, 16, 162.
[13] Burgeson, J. R.; Moore, A. L.; Boutilier, J. K.; Cerruti, N. R.; Gharaibeh, D. N.; Lovejoy, C. E.; Amberg, S. M.; Hruby, D. E.; Tyavanagimatt, S. R.; Allen Ⅲ, R. D.; Dai, D. C. Bioorg. Med. Chem. Lett. 2012, 22, 4263.
[14] Dobrikov, G. M.; Valcheva, V.; Nikolova, Y.; Ugrunova, I.; Pasheva, E.; Dimitrov, V. Eur. J. Med. Chem. 2013, 63, 468.
[15] Saeed, A.; Shah, M. S.; Larik, F. A.; Khan, S. U.; Channar, P. A.; Flörke, U.; Iqbal, J. Med. Chem. Res. 2017, 26, 1635.
[16] Wang, M. J.; Nan, X.; Feng, G.; Yu, H. T.; Hu, G. F.; Liu, Y.-Q. Ind. Crop. Prod. 2014, 55, 11.
[17] Chang, Y. N.; Zhang, J. W.; Chen, X. L..; Li, Z.; Xu, X. Y. Bioorg. Med. Chem. Lett. 2017, 27, 2641.
[18] Zhang, Q.; Zhao, B. H.; Song, Y. Y.; Hua, C. W.; Gou, X. F.; Chen B.; Zhao, J. L. Heteroat. Chem. 2015, 26, 348.
[19] Saeed, A.; Qamar R.; Fattah T. A.; Flörke, U.; Erben, M. F. Res. Chem. Intermed. 2017, 43, 3053.
[20] Murali, K.; Sparkes, H. A.; Prasad, K. J. R. Eur. J. Med. Chem. 2017, 128, 319.
[21] Sun, L. Q.; Wu, Y. B.; Liu, Y. H.; Chen, X. F.; Hu, L. X. Bioorg. Med. Chem. Lett. 2017, 27, 261.
[22] Dineshkumar, B.; Mitra, A.; Mahadevappa, M. Int. J. Phytomed. 2010, 2, 22.
[23] Wang, G. C.; Wang, J.; He, D. X.; Li, J.; Peng, Z. Y. Bioorg. Med. Chem. Lett. 2016, 26, 2806.
[24] Kong, X. Q.; Zhang, H. Z.; Cao, C. S.; Zhou, S. L.; Pang, G. S.; Shi, Y. H. Bioorg. Med. Chem. 2016, 24, 1376.
[25] Bandgar, B. P.; Adsul, L. K.; Chavan, H. V.; Jalde, S. S.; Shringare, S. N.; Shaikh, R.; Meshram, R. J.; Gacche, R. N.; Masand, V. Bioorg. Med. Chem. Lett. 2012, 22, 2539.
[26] Zhu, D. Q.; Chen, M. H.; Li, M.; Luo, B. L.; Zhao, Y.; Huang, P.; Xue, F. T.; Rapposelli, S.; Pi, R. B.; Wen, S. J. Eur. J. Med. Chem. 2013, 68, 81.
[27] Hieda, Y.; Anraku, M; Choshi, T.; Tomida, H.; Fujioka, H.; Hatae, N.; Hori, O.; Hirose, J.; Hibino, S. Bioorg. Med. Chem. Lett. 2014, 24, 3530.
[28] Börger, C.; Brütting, C.; Julich-Fruner, K. K.; Hesse, R.; Kumar, V. P.; Kutz, S. K.; Rönnefahrt, M.; Thomas, C.; Wan, B. J.; Franzblau, S. G.; Knölker, H. J. Bioorg. Med. Chem. 2017, 25(22), 6167.
[29] Ma, Q. G.; Tian, J.; Yang, J. B.; Wang, A. G.; Ji, T. F.; Wang, Y. G.; Su, Y. L. Fitoterapia 2013, 87, 1.
[30] Ty, N.; Dupeyre, G.; Chabot, G. G.; Seguin, J.; Quentin, L.; Chiaroni, A.; Tillequin, F.; Scherman, D.; Michel, S.; Cachet, X.. Eur. J. Med. Chem. 2010, 45, 3726.
[31] Akué-Gédu, R.; Nauton, L.; Théry, V.; Bain, J.; Cohen, P.; Anizon, F.; Moreau, P. Bioorg. Med. Chem. 2010, 18, 6865.
[32] Suchaud, V.; Gavara, L.; Saugues, E.; Nauton, L.; Théry, V.; Anizon, F.; Moreau, P. Bioorg. Med. Chem. 2013, 21, 4102.
[33] Lampropoulou, E.; Manioudaki, M.; Fousteris, M.; Koutsourea, A.; Nikolaropoulos, S.; Papadimitriou, E. Biomed. Pharmacother. 2011, 65, 142.
[34] Yoon, H. J.; Kong, S. Y.; Park, M. H.; Cho, Y. S.; Kim, S. E.; Shin, J. Y.; Jung, S. H.; Lee, J.; Farhanullah; Kim, H. J.; Lee, J. Bioorg. Med. Chem. 2013, 21, 7165.
[35] Thiratmatrakul, S.; Yenjai, C.; Waiwut, P.; Vajragupta, O.; Reubroycharoen, P.; Tohda, M.; Boonyarat, C. Eur. J. Med. Chem. 2014, 75, 21.
[36] Rosenker, K. M. G.; Paquette, W. D.; Johnston, P. A.; Sharlow, E. R.; Vogt, A.; Bakan, A.; Lazo, J. S.; Wipf, P. Bioorg. Med. Chem. 2015, 23, 2810.
[37] Li, Y.-J.; Yu, Y.; Jin, K.; Gao, L.-X.; Luo, T.-C..; Sheng, L.; Shao, X.; Li, J. Bioorg. Med. Chem. Lett. 2014, 24, 4125.
[38] Vo, Q. H.; Nguyen, P. H.; Zhao, B. T.; Ali, M. Y.; Choi, J. S.; Min, B. S.; Nguyen, T. H.; Woo, M. H. Fitoterapia 2015, 103, 113.
[39] Mei, W.-W.; Guo, Y.-W.; Li, J.; Cai, M.-Y.; Ma, W.-Q.; Gong, J.-X.; Wang, X.-D. Chin. J. Org. Chem. 2016, 36, 533(in Chinese). (梅雯雯, 郭跃伟, 李佳, 蔡妹艺, 马文泉, 龚景旭, 王学东, 有机化学, 2016, 36, 533.)
[40] Li, Y.-J.; Shi, X.-L.; Gao, L.-X.; Jin, K.; Sheng, L.; Wu, J.-H.; Peng, L.-N.; Li, J. Chin. J. Org. Chem. 2015, 35, 191(in Chinese). (李英俊, 史相玲, 高立信, 靳焜, 盛丽, 吴疆红, 彭丽娜, 李佳, 有机化学, 2015, 35, 191.)
[41] Li, Y.-J.; Yu, Y.; Jin, K.; Gao, L.-X.; Luo, T.-C.; Sheng, L.; Sao. X.; Li, J. Chin. J. Org. Chem. 2015, 35, 129(in Chinese). (李英俊, 于洋, 靳焜, 高立信, 罗潼川, 盛丽, 邵昕, 李佳, 有机化学, 2015, 35, 129.)
[42] Li, Y.-J.; Li, J.-Y.; Peng, L.-N.; Gao, L.-X.; Jin, K.; Sheng, L.; Zhang, N.; Wang, S.-Y.; Li, J. Chin. J. Org. Chem. 2017, 37, 485(in Chinese). (李英俊, 李继阳, 彭丽娜, 高立信, 靳焜, 盛丽, 张楠, 王思远, 李佳, 有机化学, 2017, 37, 485.)
[43] Wei, T.-B.; Chen, J.-C.; Wang, X.-C.; Yang, S.-Y. Chem. J. Chin. Univ. 1992, 13, 1217(in Chinese). (魏太保, 陈继畴, 王秀春, 杨素铀, 高等学校化学学报, 1992, 13, 1217.)
[44] Okuniewski, A.; Rosiak, D.; Chojnacki, J.; Becker, B. Acta Crystallogr. 2017, 73, 52.
[45] Saeed, A.; Ashraf, Z.; Erben, M. F.; Simpson, J. J. Mol. Struct. 2017, 1129, 283.
[46] Tabka, T.; Héron, J. F.; Gauduchon, P.; Le Talaer, J. Y.; Lancelot, J. C.; Rault, S.; Robba, M. Eur. J. Med. Chem. 1988, 23, 119.
[47] Huang, W. G.; Jiang, Y. Y.; Li, Q.; Li, J.; Li, J. Y.; Lu, W.; Cai, J. C. Tetrahedron 2005, 61, 1863.
[48] Sun, L. P.; Shen, Q.; Piao, H. H.; Ma, W. P.; Gao, L. X.; Zhang, W.; Nan, F. J.; Li, J.; Piao, H. R. Eur. J. Med. Chem. 2011, 46, 3630.
[49] Ge, Y. S.; Kamp, M. V. D.; Malaisree, M.; Liu, D.; Liu, Y.; Mulholland, A. J. J. Comput.-Aided Mol. Des. 2017, 31, 995.
[50] Wiesmann, C.; Barr, K. J.; Kung, J.; Zhu, J.; Erlanson, D. A.; Shen, W.; Fahr, B. J.; Zhong, M.; Taylor, L.; Randal, M.; McDowell, R. S.; Hansen, S. K. Nat. Struct. Mol. Biol. 2004, 11, 730.
/
| 〈 |
|
〉 |