

水相催化3,5-二烷基-4-硝基异噁唑与三氟甲基酮的亲核加成反应
收稿日期: 2017-09-29
修回日期: 2017-12-29
网络出版日期: 2018-01-26
基金资助
国家自然科学基金(Nos.21402116,21502111,21572126)、河南省重点科技攻关项目(No.172102210099)和河南省高等学校重点科研(No.15A150072)资助项目.
Catalytic Nucleophilic Addition of 3, 5-Dialkyl-4-nitroisoxazoles to Trifluoromethyl Ketones on Water
Received date: 2017-09-29
Revised date: 2017-12-29
Online published: 2018-01-26
Supported by
Project supported by the National Natural Sciences Foundation of China (Nos. 21402116, 21502111, 21572126), the Key Scientific and Technological Project of Henan Province (No. 172102210099) and the Key Science Research of Education Committee in Henan Province (No. 15A150072).
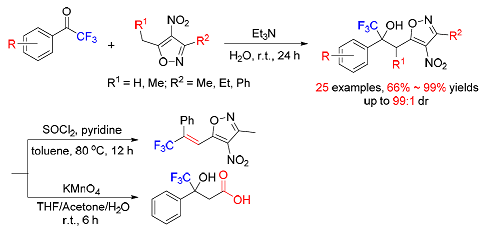
研究了水相三乙胺催化3,5-二烷基-4-硝基异噁唑与三氟甲基酮的亲核加成反应,以66%~99%的产率合成了一系列三氟甲基叔醇衍生物.通过脱水或氧化反应可有效的将目标产物转化为烯烃或羧酸类化合物.
关键词: 水相; 亲核加成; 3,5-二烷基-4-硝基异噁唑; 三氟甲基酮
王晶晶 , 李峰 , 徐妍 , 王娟 , 武紫燕 , 杨成玉 , 刘澜涛 . 水相催化3,5-二烷基-4-硝基异噁唑与三氟甲基酮的亲核加成反应[J]. 有机化学, 2018 , 38(5) : 1155 -1164 . DOI: 10.6023/cjoc201709049
The triethylamine catalyzed nucleophilic addition of 3, 5-dialkyl-4-nitroisoxazoles to trifluoromethyl ketones on water has been realized affording trifluoromethyl tertiary alcohol derivatives in 66%~99% yields. The products were easily transformed to the resulting alkenes by dehydration or acids by oxidation.

[1] (a) Welch, J. T.; Eswarakrishman, S. Fluorine in Bioorganic Chemistry, Wiley, New York, 1991.
(b) Filler, R.; Kobayashi, Y.; Yagupolskii, L. M. Organofluorine Compounds in Medicinal Chemistry and Biomedical Applications, Elsevier, Amsterdam, 1993.
(c) Banks, R. E.; Smart, B. E.; Tatlow, C. J. Organofluorine Chemistry:Principles and Comercial Applications, Springer, New York, 1994.
(d) Hiyama, T.; Kanie, K.; Kusumoto, T.; Morizawa, Y.; Shimizu, M. Organofluorine Compounds:Chemistry and Applications, Springer-Verlag, Berlin, 2000.
(e) Kirsch, P. Modern Fluoroorganic Chemistry, Wiley-VCH, Weinheim, 2005.
(f) Uneyama, K. Organofluorine Chemistry, Blackwell, Oxford, 2007.
(g) Ojima, I. Fluorine in Medicinal Chemistry and Chemical Biology, Wiley-Blackwell, Chichester, 2009.
[2] (a) Kirk, K. L. J. Fluorine Chem. 2006, 127, 1013.
(b) Mîller, K.; Faeh, C.; Diederich, F. Science 2007, 317, 1881.
(c) Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 320.
(d) Hagmann, W. K. J. Med. Chem. 2008, 51, 4359.
(e) Berger, R.; Resnati, G.; Metrangolo, P.; Weber, E.; Hulliger, J. Chem. Soc. Rev. 2011, 40, 3496.
(f) Ojima, I. J. Org. Chem. 2013, 78, 6358.
(g) Fujiwara, T.; O_Hagan, D. J. Fluorine Chem. 2014, 167, 16.
(h) Wang, J.; Sánchez-Roselló, M.; Aceña, J. L.; Pozo, C.; Sorochinsky, A. E.; Fustero, S.; Soloshonok, V. A.; Liu, H. Chem. Rev. 2014, 114, 2432.
(i) Gillis, E. P.; Eastman, K. J.; Hill, M. D.; Donnelly, D. J.; Meanwell, N. A. J. Med. Chem. 2015, 58, 8315.
[3] (a) Pierce, M. E.; Rodney, L. P. J.; Radesca, L. A.; Lo, Y. S.; Silverman, S.; Moore, J. R.; Islam, Q.; Choudhury, A.; Fortunak, J. M. D.; Nguyen, D.; Luo, C.; Morgan, S. J.; Davis, W. P.; Confalone, P. N.; Chen, C.-Y.; Tillyer, R. D.; Frey, L.; Tan, L.; Xu, F.; Zhao, D.; Thompson, A. S.; Corley, E. G.; Grabowski, E. J. J.; Reamer, R.; Reider, P. J. J. Org. Chem. 1998, 63, 8536.
(b) Corbett, J. W.; Ko, S. S.; Rodgers, J. D.; Gearhart, L. A.; Magnus, N. A.; Bacheler, L. T.; Diamond, S.; Jeffrey, S.; Klabe, R. M.; Cordova, B. C.; Garber, S.; Logue, K.; Trainor, G. L.; Anderson, P. S.; Erickson-Viitanen, S. K. J. Med. Chem. 2000, 43, 2019.
[4] Schenck, H. A.; Lenkowski, P. W.; Choudhury-Mukherjee, I.; Ko, S.-H.; Stables, J. P.; Patel, M. K.; Brown, M. L. Bioorg. Med. Chem. 2004, 12, 979.
[5] Fandrick, D. R.; Reeves, J. T.; Bakonyi, J. M.; Nyalapatla, P. R.; Tan, Z.; Niemeier, O.; Akalay, D.; Fandrick, K. R.; Wohlleben, W.; Ollenberger, S.; Song, J. J.; Sun, X.; Qu, B.; Haddad, N.; Sanyal, S.; Shen, S.; Ma, S.; Byrne, D.; Chitroda, A.; Fuchs, V.; Narayanan, B. A.; Grinberg, N.; Lee, H.; Yee, N.; Brenner, M.; Senanayake, C. H. J. Org. Chem. 2013, 78, 3592.
[6] (a) Betageri, R.; Zhang, Y.; Zindell, R. M.; Kuzmich, D.; Kirrane, T. M.; Bentzien, J.; Cardozo, M.; Capolino, A. J.; Fadra, T. N.; Nelson, R. M.; Paw, Z.; Shih, D.-T.; Shih, C.-K.; Zuvela-Jelaska, L.; Nebozny, G.; Thomson, D. Bioorg. Med. Chem. Lett. 2005, 15, 4761.
(b) Barker, M.; Clackers, M.; Copley, R.; Demaine, D. A.; Humphreys, D.; Inglis, G. G. A.; Johnston, M. J.; Jones, H. T.; Haase, M. V.; House, D.; Loiseau, R.; Nisbet, L.; Pacquet, F.; Skone, P. A.; Shanahan, S. E.; Tape, D.; Vinader, V. M.; Washington, M.; Uings, I.; Upton, R.; McLay, I. M.; Macdonald, S. J. F. J. Med. Chem. 2006, 49, 4216.
[7] (a) Sani, M.; Viani, F.; Binda, M.; Zaffaroni, N.; Zanda, M. Lett. Org. Chem. 2005, 2, 447.
(b) Betageri, R.; Gilmore, T.; Kuzmich, D.; Kirrane, T. M.; Bentzien, J.; Wiedenmayer, D.; Bekkali, Y.; Regan, J.; Berry, A.; Latli, B.; Kukulka, A. J.; Fedra, T. N.; Nelson, R. N.; Goldrick, S.; Zuvela-Jelaska, L.; Souza, D.; Pelletier, J.; Dinallo, R.; Panzenbeck, M.; Torcellini, C.; Lee, H.; Pack, E.; Harcken, C.; Nabozny, G.; Thomson, D. S. Bioorg. Med. Chem. Lett. 2011, 21, 6842.
[8] Carceller, E.; Merlos, M.; Giral, M.; Balsa, D.; Garciía-Rafanell, J.; Forn, J. J. Med. Chem. 1996, 39, 487.
[9] For reviews, see:(a) Nie, J.; Guo, H.-C.; Cahard, D.; Ma, J.-A. Chem. Rev. 2011, 111, 455.
(b) Kelly, C. B.; Mercadante, M. A.; Leadbeater, N. E. Chem. Commun. 2013, 49, 11133.
[10] For selected examples:(a) Hara, N.; Tamura, R.; Funahashi, Y.; Nakamura, S. Org. Lett. 2011, 13, 1662.
(b) Zhang, G.-W.; Meng, W.; Ma, H.; Nie, J.; Zhang, W.-Q.; Ma, J.-A. Angew. Chem., Int. Ed. 2011, 50, 3538.
(c) Cui, H.-F.; Wang, L.; Yang, L.-J.; Nie, J.; Zheng, Y.; Ma, J.-A. Tetrahedron 2011, 67, 8470.
(d) Li, X.-J.; Xiong, H.-Y.; Hua, M.-Q.; Nie, J.; Zheng, Y.; Ma, J.-A. Tetrahedron Lett. 2012, 53, 2117.
(e) Zheng, Y.; Xiong, H.-Y.; Nie, J.; Hua, M.-Q.; Ma, J.-A. Chem. Commun. 2012, 48, 4308.
(f) Luo, R.; Li, K.; Hu, Y.; Tang, W. Adv. Synth. Catal. 2013, 355, 1297.
(g) Zong, H.; Huang, H.; Bian, G.; Song, L. J. Org. Chem. 2014, 79, 11768.
(h) Wang, L.; Liu, N.; Dai, B.; Ma, X.; Shi, L. RSC Adv. 2015, 5, 10089.
(i) Jamal, Z.; Teo, Y.-C. RSC Adv. 2015, 5, 26949.
(j) Zhang, D.; Tanaka, F. Adv. Synth. Catal. 2015, 357, 3458.
(k) Tao, R.; Yin, X.-J.; Wang, K.-H.; Niu, Y.-Z.; Wang, Y.-L.; Huang, D.-F.; Su, Y.-P.; Wang, J.-X.; Hu, Y.-L.; Fu, Y.; Du, Z.-Y. Chin. Chem. Lett. 2015, 26, 1046.
(l) Jing, Z.; Bai, X.; Chen, W.; Zhang, G.; Zhu, B.; Jiang, Z. Org. Lett. 2016, 18, 260.
(m) Lutete, L. M.; Miyamoto, T.; Ikemoto, T. Tetrahedron Lett. 2016, 57, 1220.
(n) Cook, A. M.; Wolf, C. Angew. Chem., Int. Ed. 2016, 55, 2929.
(o) Lee, K.; Silverio, D. L.; Torker, S.; Robbins, D. W.; Haeffner, F.; Mei, F. W.; Hoveyda, A. H. Nat. Chem. 2016, 8, 768.
(p) Matador, E.; Monge, D.; Fernández, R.; Lassaletta, J. M. Green Chem. 2016, 18, 4042.
(q) Mszar, N. W.; Mikus, M. S.; Torker, S.; Haeffner, F.; Hoveyda, A. H. Angew. Chem., Int. Ed. 2017, 56, 8736.
(r) Noda, H.; Amemiya, F.; Weidner, K.; Kumagai, N.; Shibasaki, M. Chem. Sci. 2017, 8, 3260.
(s) Bai, X.; Zeng, G.; Shao, T.; Jiang, Z. Angew. Chem., Int. Ed. 2017, 56, 3684.
(t) Zheng, Y.; Tan, Y.; Harms, K.; Marsch, M.; Riedel, R.; Zhang, L.; Meggers, E. J. Am. Chem. Soc. 2017, 139, 4322.
[11] Bandini, M.; Sinisi, R. Org. Lett. 2009, 11, 2093.
[12] Zhang, Y.; Wei, B.-W.; Zou, L.-N.; Kang, M.-L.; Luo, H.-Q.; Fan, X.-L. Tetrahedron 2016, 72, 2472.
[13] Czerwinski, P.; Molga, E.; Cavallo, L.; Poater, A.; Michalak, M. Chem. Eur. J. 2016, 22, 8089.
[14] (a) Grieco, P. A. Organic Synthesis in Water, Blackie, London, 1998.
(b) Lindstrom, U. M. Organic Reactions in Water:Principles, Strategies and Applications, Blackwell, Oxford, U. K., 2007.
(c) Li, C.-J.; Chan, T.-H. Comprehensive Organic Reactions in Aqueous Media, Wiley, New York, 2007.
[15] For selected reviews:(a) Lindstrom, U. M. Chem. Rev. 2002, 102, 2751.
(b) Head-Gordon, T.; Hura, G. Chem. Rev. 2002, 102, 2651.
(c) Hayashi, Y. Angew. Chem., Int. Ed. 2006, 45, 8103.
(d) Mase, N.; Barbas Ⅲ, C. F. Org. Biomol. Chem. 2010, 8, 4043.
[16] For selected reviews:(a) Li, C.-J. Chem. Rev. 2005, 105, 3095.
(b) Li, C.-J.; Chen, L. Chem. Soc. Rev. 2006, 35, 68.
(c) Chanda, A.; Fokin, V. V. Chem. Rev. 2009, 109, 725.
(d) Butler, R. N.; Coyne, A. G. Chem. Rev. 2010, 110, 6302.
(e) Simon, M.-O.; Li, C.-J. Chem. Soc. Rev. 2012, 41, 1415.
(f) Gawande, M. B.; Bonifacio, V. D. B.; Luque, R.; Branco, P. S.; Varma, R. S. Chem. Soc. Rev. 2013, 42, 5522.
(g) Levin, E.; Ivry, E.; Diesendruck, C. E.; Lemcoff, N. G. Chem. Rev. 2015, 115, 4607.
(h) Butler, R. N.; Coyne, A. G. Org. Biomol. Chem. 2016, 14, 9945.
[17] For selected examples:(a) Phippen, C. B. W.; Beattie, J. K.; McErlean, C. S. P. Chem. Commun. 2010, 46, 8234.
(b) Fu, X.-P.; Liu, L.; Wang, D.; Chen, Y.-J.; Li, C.-J. Green. Chem. 2011, 13, 549.
(c) Sakakura, A.; Koshikari, Y.; Akakura, M.; Ishihara, K. Org. Lett. 2011, 14, 30.
(d) Paladhi, S.; Chauhan, A.; Dhara, K.; Tiwari, A. K.; Dash, J. Green. Chem. 2012, 14, 2990.
(e) Islam, S.; Larrosa, I. Chem. Eur. J. 2013, 19, 15093.
(f) Sengoden, M.; Punniyamurthy, T. Angew. Chem. Int. Ed. 2013, 52, 572.
(g) Paladhi, S.; Bhati, M.; Panda, D.; Dash, J. J. Org. Chem. 2014, 79, 1473.
(h) Thakur, P. B.; Meshram, H. M. RSC Adv. 2014, 4, 5343.
(i) Thakur, P. B.; Meshram, H. M. RSC Adv. 2014, 4, 6019.
(j) Yu, J.-S.; Liu, Y.-L.; Tang, J.; Wang, X.; Zhou, J. Angew. Chem. Int. Ed. 2014, 53, 9512.
(k) Yang, F.; Klumphu, P.; Liang, Y.-M.; Lipshutz, B. H. Chem. Commun. 2014, 50, 936.
(l) Zhang, F.-Z.; Tian, Y.; Li, G.-X.; Qu, J. J. Org. Chem. 2015, 80, 1107.
(m) SaiPrathima, P.; Srinivas, K.; Mohan Rao, M. Green. Chem. 2015, 17, 2339.
(n) Bhattacharjya, A.; Klumphu, P.; Lipshutz, B. H. Nat. Commun. 2015, 6, 7401.
(o) Cho, B. S.; Chung, Y. K. Chem. Commun. 2015, 51, 14543.
(p) Xiao, J.; Wen, H.; Wang, L.; Xu, L.; Hao, Z.; Shao, C.-L.; Wang, C.-Y. Green Chem. 2016, 18, 1032.
(q) Zhang, Y.; Wei, B.-W.; Lin, H.; Zhang, L.; Liu, J.-X.; Luo, H.-Q.; Fan, X.-L. Green Chem. 2015, 17, 3266.
(r) Ren, N.; Nie, J.; Ma, J.-A. Green Chem. 2016, 18, 6609.
[18] (a) Wang, J.; Kong, W.-G.; Li, F.; Liu, J.; Shen, Q.; Liu, L.; Zhao, W.-X. Org. Biomol. Chem. 2015, 13, 5399.
(b) Li, F.; Wang, J.; Xu, M.; Zhao, X.; Zhou, X.; Zhao, W.-X.; Liu, L. Org. Biomol. Chem. 2016, 14, 3981.
(c) Wang, J.; Li, F.; Shen, Q.; Pei, W.; Zhao, W.-X.; Liu, L. Synthesis 2016, 48, 441.
(d) Wang, J.; Li, F.; Xie, H.; Xu, M.; Zhao, X.; Liu, L.; Zhao, W.-X. Appl. Organomet. Chem. 2017, 31, e3545.
[19] CCDC 1558716(3a) and CCDC 1559048(5) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Center via www.ccdc.cam.ac.uk/data_request/cif.
[20] (a) Creary, X. J. Org. Chem. 1987, 52, 5026.
(b) Chong, J. M.; Mar, E. K. J. Org. Chem. 1991, 56, 893.
(c) Prakash, G. K. S.; Panja, C.; Vaghoo, H.; Surampudi, V.; Kultyshev, R.; Mandal, M.; Rasul, G.; Mathew, T.; Olah, G. A. J. Org. Chem. 2006, 71, 6806.
(d) Cheng, H.; Pei, Y.; Leng, F.; Li, J.; Liang, A.; Zou, D.; Wu, Y.; Wu, Y. Tetrahedron Lett. 2013, 54, 4483.
[21] (a) Adamo, M. F. A.; Duffy, E. F. Org. Lett. 2006, 8, 5157.
(b) Adamo, M. F. A.; Suresh, S. Tetrahedron 2009, 65, 990.
(c) Zhang, J.; Liu, X.; Ma, X.; Wang, R. Chem. Commun. 2013, 49, 9329.
[22] Gao, J.-R.; Wu, H.; Xiang, B.; Yu, W.-B.; Han, L.; Jia, Y.-X. J. Am. Chem. Soc. 2013, 135, 2983.
[23] Fiandra, C. D.; Piras, L.; Fini, F.; Disetti, P.; Moccia, M.; Adamo, M. F. A. Chem. Commun. 2012, 48, 3863.
[24] McBee, E. T.; Kim, Y. S.; Braendlin, H. P. J. Am. Chem. Soc. 1962, 84, 3154.
/
| 〈 |
|
〉 |