

吗啉核苷类似物及其磺胺衍生物的合成及初步抗牛病毒性腹泻病毒(BVDV)活性研究
收稿日期: 2017-12-08
修回日期: 2018-01-10
网络出版日期: 2018-01-26
基金资助
国家自然科学基金(No.21372207)资助项目.
Synthesis and Preliminary Anti-bovine Viral Diarrhea Virus (BVDV) Activity Evaluation of Morpholine Nucleoside Analogues and Their Sulfonamide Derivatives
Received date: 2017-12-08
Revised date: 2018-01-10
Online published: 2018-01-26
Supported by
Project supported by the National Natural Science Foundation of China (No. 21372207).
华迎春 , 贺斌 , 秦之焱 , 王松 , 刘慧萍 , 刘丰五 . 吗啉核苷类似物及其磺胺衍生物的合成及初步抗牛病毒性腹泻病毒(BVDV)活性研究[J]. 有机化学, 2018 , 38(5) : 1147 -1154 . DOI: 10.6023/cjoc201712017
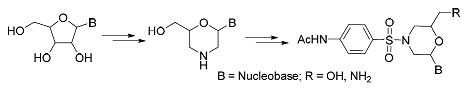
A series of morpholine nucleoside analogues were synthesized starting from ribonucleosides, and then the morpholine nucleoside sulfonamide derivatives were obtained by treated with N-acetylsulfanilyl chloride. The obtained compounds were confirmed by HRMS, 1H NMR and 13C NMR spectral analysis. The bovine kidney cells infected with bovine viral diarrhea virus (BVDV) were used as models to screen the antiviral activity of the target compounds. But the results did not show desired potent antiviral activity.
[1] Gao, Y. X.; Hu, J.; Ju, Y. Acta Chim. Sinica 2016, 74, 312(in Chinese). (高玉霞, 胡君, 巨勇, 化学学报, 2016, 74, 312.)
[2] Nie, B.; Jin, C. F.; Zhong, W. H.; Ren, Q. Y.; Zhang, Y. J.; Zhang, J. Chin. J. Org. Chem. 2017, 37, 2818(in Chinese). (聂飚, 金传飞, 钟文和, 任青云, 张英俊, 张霁, 有机化学, 2017, 37, 2818.)
[3] Ilardi, E. A.; Vitaku, E.; Njardarson, J. T. J. Med. Chem. 2014, 57, 2832.
[4] Wulf, N. R.; Matuszewski, K. A. Am. J. Health-Syst. Pharm. 2013, 70, 1483.
[5] Gawin, R.; De, C. E.; Naesens, L.; Koszytkowska-Stawinska, M. Bioorg. Med. Chem. 2008, 16, 8379.
[6] Zhang, N.; Tan, C.; Cai, P.; Jiang, Y.; Zhang, P.; Zhao, Y. Tetrahedron Lett. 2008, 49, 3570.
[7] Pattanayak, S.; Paul, S.; Sinha, N. B. S. Nucleosides, Nucleotides Nucleic Acids 2012, 31, 763.
[8] Hickman, D. T.; Sreenivasachary, N.; Lehn, J.-M. Helv. Chim. Acta 2008, 91, 1.
[9] Nicolaou, K. C.; Adsool, V. A.; Hale, C. R. Org. Lett. 2010, 12, 1552
[10] Ermolinski, B. S.; Mikhailov, S. N. Russ. J. Bioorg. Chem. 2000, 26, 429.
[11] Tronchet, J. M. J.; Zsély, M.; Cabrini, D.; Jorand, C.; Barbalat-Rey, F.; Komaromi, I; Ricca, A.; Geoffroy, M. Nucleosides Nucleotides 2006, 12, 615.
[12] Hudson, R. H. E.; Li, G.; Tse, J. Tetrahedron Lett. 2002, 43, 1381.
[13] Paul, S.; Pattanayak, S.; Sinha, S. Tetrahedron Lett. 2014, 55, 1072.
[14] Tichenor, M. S.; Trzupek, J. D.; Kastrinsky, D. B.; Hwang, I.; Shiga, F.; Boger, D. L. J. Am. Chem. Soc. 2006, 128, 15683.
[15] Bialecki, J. B.; Yuan, L. H.; Gong, B. Tetrahedron 2007, 63, 5460.
[16] Wang, X. J.; Hou, M. L.; Chen, L. G.; Meng, Y.; Li, Y. Chemistry 2004, 67, 418(in Chinese). (王晓季, 侯曼玲, 陈立功, 孟祎, 李阳, 化学通报, 2004, 67, 418.)
[17] Li, J.; Lowary, T. L. Org. Lett. 2008, 10, 881.
[18] Vasina, V. A; Korovina, D. Y.; Razin, V. V. Russ. J. Org. Chem. 2016, 52, 932.
[19] Fringuelli, F.; Pizzo, F.; Rucci, M.; Vaccaro, L. J. Org. Chem. 2003, 68, 7041.
[20] Salunkhel, A. M.; Brown, H. C. Tetrahedron Lett. 1995, 36, 7987.
[21] Loos, M. D.; Esch, J. H. V.; Kellogg, R. M.; Feringa, B. L. Tetrahedron 2007, 63, 7285.
/
| 〈 |
|
〉 |