

偶氮桥联的1,3,5-多取代三唑类化合物的合成及抑菌活性
收稿日期: 2017-10-09
修回日期: 2018-01-29
网络出版日期: 2018-02-06
基金资助
国家自然科学基金(No.31272076)和国家科技支撑计划(No.2015BAK45B01)资助项目.
Synthesis and Fungicidal Activity of Bridging Ligand Compounds of Azo with 1, 3, 5-Substitued Triazole
Received date: 2017-10-09
Revised date: 2018-01-29
Online published: 2018-02-06
Supported by
Project supported by the National Natural Science Foundation of China (No. 31272076) and the National Key Technology Support Program of China (No. 2015BAK45B01).
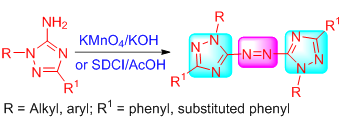
以1,3,5-三取代-1,2,4-三唑类化合物为原料,在KMnO4或二氯异氰尿酸钠(SDCI)作用下,发生自身氧化偶联反应,合成了16个未见文献报道的偶氮桥联的1,3,5-多取代三唑类化合物.产物结构经1H NMR、13C NMR、IR、HRMS及X射线单晶衍射得以证实,并采用生长速率法对所有化合物进行了离体抑菌活性测试.结果表明:在浓度为50 μg/mL时,大部分化合物对所选六种病菌具有一定的抑制活性,其中1,1'-二正丁基-3,3'-二(2''-氯苯基)-5,5'-偶氮二(1,2,4-三唑)(3h)、1,1'-二丙基-3,3'-二(4''-氯苯基)-5,5'-偶氮二(1,2,4-三唑)(3k)对瓜果腐霉菌的抑制率接近三唑酮,而1,1'-二丙基-3,3'-二(2''-氯苯基)-5,5'-偶氮二(1,2,4-三唑)(3g)的抑制率要高于三唑酮.该类化合物反应操作简单、条件温和,对新农药创制及合成具有借鉴意义.
李长胜 , 邹玉龙 , 贾长青 , 覃兆海 , 马永强 . 偶氮桥联的1,3,5-多取代三唑类化合物的合成及抑菌活性[J]. 有机化学, 2018 , 38(6) : 1500 -1506 . DOI: 10.6023/cjoc201710011
Sixteen bridging ligand compounds of azo with 1,3,5-substitued triazole had been synthesized from 1,3,5-trisub-stituted-1,2,4-triazoles by the oxidative coupling reaction using potassium permanganate or sodium dichloroisocyamirate as the oxidant. The structures of the target compounds were determined by 1H NMR, 13C NMR, IR, MS, HRMS and X-ray diffraction. They were tested for in vitro fungicidal activities by the mycelium growth rate method. The results indicated that most of the title compounds had a certain inhibitory activity at the concentration of 50 μg/mL. Especially, the inhibition rate of 1,2-bis(1-butyl-3-(2-chlorophenyl)-1H-1,2,4-triazol-5-yl)diazene (3h) and 1,2-bis(3-(4-chlorophenyl)-1-propyl-1H-1,2,4-triazol-5-yl)diazene (3k) were close to that of triadimefon when against Pythium aphanidermatum, while the inhibition rate of 1,2-bis(3-(2-chlorophenyl)-1-propyl-1H-1,2,4-triazol-5-yl)diazene (3g) was higher than that of riadimefon. The reactions of these compounds were simple and mild, which was of reference significance to the creation and synthesis of new pesticides.

Key words: triazole; azo compounds; oxidative coupling; fungicidal activities
[1] Stefaskan, J.; Struga, M.; Tyski, S.; Kossakowski, J.; Dobosz, M. Pol. J. Microbiol. 2008, 57, 179.
[2] Wang, B.-L.; Shi, Y.-X.; Ma, Y.; Liu, X.-H.; Li, Y.-H.; Song. H.-B.; Li, B.-J.; Li, Z.-M. J. Agric. Food Chem. 2010, 58, 551.
[3] Plech, T.; Wujec, M.; Kosikowska, U.; Malm, A.; Kapron, B. Eur. J. Med. Chem. 2012, 47, 580.
[4] Barbuceanu, S.-F.; Saramet, G.; Almajan, G. L.; Draghici, C.; Barbuceanu, F.; Bancescu, G. Eur. J. Med. Chem. 2012, 49, 417.
[5] Lin, X.-F.; Tan, Z.; Liu, Y.; He, Y.-J.; Bao, X.-P. Chin. J. Org. Chem. 2013, 33, 353(in Chinese). (林选福, 谭赞, 刘勇, 贺银菊, 鲍小平, 有机化学, 2013, 33, 353.)
[6] Liu, J.-H.; Liu, Y.; Jian, J.-Y.; Bao, X.-P. Chin. J. Org. Chem. 2013, 33, 370(in Chinese). (刘军虎, 刘勇, 蹇军友, 鲍小平, 有机化学, 2013, 33, 370.)
[7] Bao, X.-P.; Lin, X.-F.; Jian, J.-Y.; Zhang, F.; Zou, L.-B. Chin. J. Org. Chem. 2013, 33, 995(in Chinese). (鲍小平, 林选福, 蹇军友, 张峰, 邹林波, 有机化学, 2013, 33, 995.)
[8] Wang, B.-L.; Zhang, Y.; Liu, X.-H.; Zhang, L.-Y.; Zhan, Y.-Z.; Zhang, X.; Wang, L.-Z.; Li, Y.-H.; Li, Z.-M. Phosphorus, Sulfur Silicon Relat. Elem. 2017, 192, 34
[9] Fan, Z.-J; Yang, Z.-K.; Zhang, H.-K.; Mi, N.; Wang, H.; Cai, F.; Zou, X.; Zheng, Q.-X.; Song, H. J. Agric. Food Chem. 2010, 58, 2630.
[10] Song, X.-J.; Wang, S.; Tan, X.-H.; Wang, Z.-Y.; Wang, Y.-G. Chin. J. Org. Chem. 2007, 27, 72(in Chinese). (宋新建, 王胜, 谭小红, 王子云, 汪炎钢, 有机化学, 2007, 27, 72.)
[11] Deng, X.-Q.; Song, M.-X.; Wei, C.-X.; Li, F.-N.; Quan, Z. S. Med. Chem. 2010, 6, 313.
[12] Drabent, K.; Bialonska, A.; Ciunik, Z. Inorg. Chem. Commun. 2004, 7, 224.
[13] Pan, L.; Chen, Y.-W.; Liu, Z.; Li, Y.-H.; Li, Z.-M. Chin. J. Org. Chem. 2013, 33, 542(in Chinese). (潘里, 陈有为, 刘卓, 李永红, 李正名, 有机化学, 2013, 33, 542.)
[14] Sumangala, V.; Poojary, B.; Chidananda, N.; Arulmoli, T.; Shenoy, S. Eur. J. Med. Chem. 2012, 54, 59.
[15] Mavrova, A. T.; Wesselinova, D.; Tsenov, Y. A.; Denkova, P. Eur. J. Med. Chem. 2009, 44, 63.
[16] Abdel-Wahab, B. F.; Abdel-Latif, E.; Mohamed, H. A.; Awad, G. E. A. Eur. J. Med. Chem. 2012, 52, 263.
[17] Almasirad, S. A.; Tabatabai, M.; Faizi, A.; Kebriaeezadeh, N.; Mehrabi, A.; Dalvandi, A. S. Bioorg. Med. Chem. Lett. 2004, 14, 6057.
[18] Amir, M.; Shikha, K. Eur. J. Med. Chem. 2004, 39, 535
[19] Il'inykh, E.-S.; Kim, D.-G. Chem. Heterocycl. Compd. 2011, 47, 636.
[20] Mioc, M.; Soica, C.; Bercean, V.; Avram, S.; Balan-Porcarasu, M.; Coricovac, D.; Ghiulai, R.; Muntean, D.; Andrica, F.; Dehelean, C.; Spandidos, D.-A.; Tsatsakis, A.-M.; Kurunczi, L. Int. J. Oncol. 2017, 50, 1175.
[21] Tumosiene, I.; Jonuskiene, I.; Kantminiene, K.; Beresnevicius, Z.-J. Monatsh. Chem. 2014, 145, 319.
[22] Xu, H.; Zeng, X.-W. Bioorg. Med. Chem. Lett. 2010, 20, 4193.
[23] Sayed, A. Z.; Aboul-Fetouh, M. S.; Nassar, H. S. J. Mol. Struct. 2012, 1010, 146.
[24] Patel, N. B.; Sharma, R. D. Synth. Commun. 2013, 43, 1250.
[25] Kuznetsov, D. N.; Ruchkina, A. G.; Kobrakov, K. I. Chem. Heterocycl. Compd. 2011, 47, 441.
[26] Subramanyam, S.; Raja, S.; Jayaveera, K. N. J. Pharm. Chem. 2012, 6, 10.
[27] Li, Z.; Qian, X.-H.; Ye, Z.-J.; Xu, X.-Y.; Shao, X.-S.; Tao, L.-M.; Song, G.-H.; Xu, Z.-P. CN 102464653, 2012.
[28] Elsharabasy, F. S.; Gomha, S. M.; Farghaly, T. A.; Elzahabi, H. S. A. Molecules 2017, 22, 319.
[29] Qin, Z.-H.; Ma, Y.-Q.; Su, W.-C.; Wang, L.; Zhang, Z.; Zhao, B.-B.; Fang, J.-S. CN 101821232, 2010.
[30] Qin, Z.-H.; Mu, C.-W.; Li, N.; Fu, B.; Zhang, S.-S.; Xiao, Y.-M. CN 1939128, 2006.
[31] Su, W.-C.; Zhou, Y.-H.; Ma, Y.-Q.; Wang, L.; Zhang, Z.; Rui, C.-H.; Duan, H.-X.; Qin, Z.-H. J. Agric. Food Chem. 2012, 60, 5028.
[32] Qin, Z.-H.; Ma, Y.-Q.; Zhou, Y.-H.; Xu, Y.; Jia, C.-Q.; Zhou, Z.-Y.; Yang, D.-Y. CN 102863360, 2013.
[33] Qin, Z.-H.; Ma, Y.-Q.; Su, W.-C.; Zhao, B.-B.; Fang, J.-S. WO 2013003977, 2013.
[34] Jia, C.-Q.; Yang, D.-Y.; Che, C.-L.; Ma, Y.-Q.; Rui, C.-H.; Yan, X.-J.; Qin, Z.-H. Chem. J. Chin. Univ. 2016, 37, 892(in Chinese). (贾长青, 杨冬燕, 车传亮, 马永强, 芮昌辉, 闫晓静, 覃兆海, 高等学校化学学报, 2016, 37, 892.)
[35] Jia, C.-Q.; Su, W.-C.; Xu, Y.-J.; Liu, J.-P.; Qin, Z.-H. Chin. J. Org. Chem. 2016, 36, 830(in Chinese). (贾长青, 苏旺苍, 徐彦军, 刘吉平, 覃兆海, 有机化学, 2016, 36, 830.)
[36] Venugopal, T.; Jean'ne, M. S. J. Am. Chem. Soc. 2011, 133, 19982.
[37] Li, Y.-C.; Qi, C.; Li, S.-H.; Zhang, H.-J.; Sun, C.-H.; Yu, Y.-Z.; Pang, S.-P. J. Am. Chem. Soc. 2010, 132, 12172.
[38] Allen, F. H.; Kennard, O.; Watson, D. G.; Brammer, L.; Orpen, A. G.; Taylor, R. J. Chem. Soc., Perkin Trans. 21987, S1.
/
| 〈 |
|
〉 |