

1,5-溴三氯甲基化产物及三取代苯乙烯的E-Z异构化
收稿日期: 2017-12-02
修回日期: 2018-01-30
网络出版日期: 2018-02-06
基金资助
国家自然科学基金(No.21572099,21332005)资助项目.
E-Z Isomerization of 1, 5-Bromotrichloromethylation Reaction Products and Trisubstituted Styrenes
Received date: 2017-12-02
Revised date: 2018-01-30
Online published: 2018-02-06
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21572099, 21332005).
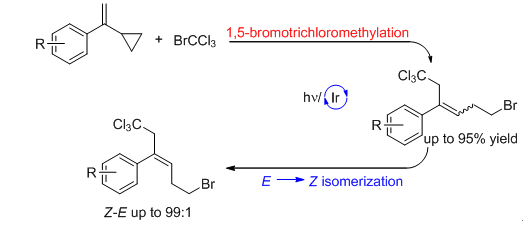
在可见光照射下,以Ir[dF(CF3) ppy]2(dtbbpy) PF6为光催化剂,α-环丙烷苯乙烯通过自由基链式反应机理进行1,5-溴三氯甲基化反应,生成三取代的苯乙烯类化合物,起始的Z/E比例为30∶70.当反应化合物进一步进行光照的时候,产物的Z/E比例最高可达99∶1.利用这一方法,成功合成了一系列的含溴三氯甲基的三取代苯乙烯化合物,产率从良好到优秀,都以Z构型为主.通过量子产率实验和荧光淬灭实验,提出了一个串联的反应历程,包含可见光引发的自由基链式反应及光催化剂催化的E-Z异构化反应.在此基础上,直接对容易制备的E构型的三取代苯乙烯类化合物进行可见光条件下的构型翻转,获得Z构型产物.
李进 , 陈靖之 , 黄文浩 , 程旭 . 1,5-溴三氯甲基化产物及三取代苯乙烯的E-Z异构化[J]. 有机化学, 2018 , 38(6) : 1507 -1515 . DOI: 10.6023/cjoc201712002
1,5-Bromotrichloromethylation of α-cyclopropylstyrenes via a radical chain pathway was achieved with Ir[dF(CF3)ppy]2(dtbbpy)PF6 as a photoinitiator under visible-light irradiation to give trisubstituted styrenes with Z/E ratio of 30:70. When the reaction mixture was further irradiated, the Z/E ratio could be reversed and increased to 99:1, probably via an energy-transfer pathway involving the Ir photocatalyst. This visible-light-induced catalytic isomerization protocol could also be applied to trisubstituted styrenes to obtain products with Z/E ratios up to 99:1.

Key words: photochemistry; radical; alkene; isomerization
[1] (a) Yale, H. L. J.Med.Chem. 1959, 1, 121.
(b) Hagmann, W. K. J.Med.Chem. 2008, 51, 4359.
(c) Tomashenko, O. A.; Grushin, V. V. Chem.Rev. 2011, 111, 4475.
(d) Mullard, A. Nat.Rev.Drug.Discovery 2013, 12, 87.
[2] (a) Ma, J.-A.; Cahard, D. Chem.Rev. 2008, 108, PR1.
(b) Chen, P.; Liu, G. Synthesis 2013, 45, 2919.
(c) Liu, H.; Gu, Z.; Jiang, X. Adv.Synth.Catal. 2013, 355, 617.
(d) Chu, L.; Qing, F.-L. Acc.Chem.Res. 2014, 47, 15132.
(e) Zhang, C. Org.Biomol.Chem. 2014, 12, 6580.
(f) Alonso, C.; Martinez de Marigorta, E.; Rubiales, G.; Palacios, F. Chem. Rev. 2015, 115, 1847.
(g) Liu, X.; Xu, C.; Wang, M.; Liu, Q. Chem.Rev. 2015, 115, 683.
[3] (a) Hofheinz, W.; Oberhansli, W. E. Helv. Chim.Acta 1977, 60, 660.
(b) Unson, M. D.; Rose, C. B.; Faulkner, D. J.; Brinen, L. S.; Steiner, J. R.; Clardy, J. J.Org.Chem. 1993, 58, 6336.
(c) Orjala, J.; Gerwick, W. H. J.Nat.Prod. 1996, 59, 427.
(d) Fu, X.; Zeng, L.-M.; Su, J.-Y.; Pais, M. J.Nat.Prod. 1997, 60, 695.
(e) MacMillan, J. B.; Trousdale, E. K.; Molinski, T. F. Org.Lett. 2000, 2, 2721.
(f) Orsini, M. A.; Pannell, L. K.; Erickson, K. L. J.Nat.Prod. 2001, 64, 572.
[4] (a) Hutt, M. P.; Elslager, E. F.; Werbel, L. M. J. Heterocycl.Chem. 1970, 7, 511.
(b) Werbel, L. M.; Elslager, E. F.; Hess, C.; Hutt, M. P. J.Med. Chem. 1987, 30, 1943.
(c) Fujisawa, T.; Ito, T.; Fujimoto, K.; Shimizu, M.; Wynberg, H.; Staring, E. G. J. Tetrahedron Lett. 1997, 38, 1593.
(d) Huffman, M. A.; Reider, P. J. Tetrahedron Lett. 1999, 40, 831.
(e) Bringmann, G.; Feineis, D.; God, R.; Peters, K.; Peters, E.-M.; Scholz, J.; Riederer, F.; Moser, A. Biorg.Med.Chem. 2002, 10, 2207.
[5] Wallentin, C.-J.; Nguyen, J. D.; Finkbeiner, P.; Stephenson, C. R. J. J.Am.Chem.Soc. 2012, 134, 8875.
(b) Arceo, E.; Montroni, E.; Melchiorre, P. Angew.Chem., Int. Ed. 2014, 53, 12064.
(c) Liu, Y.; Zhang, J.-L.; Song, R.-J.; Li, J.-H. Eur.J.Org. Chem. 2014, 6, 1177.
(d) Franz, J. F.; Kraus, W. B.; Zeitler, K. Chem.Commun. 2015, 51, 8280.
[6] Huo, H.; Wang, C.; Harms, K.; Meggers, E. J. Am.Chem.Soc. 2015, 137, 9551.
[7] Li, X.; Wu, J.; Chen, L.; Zhong, X.; He, C.; Zhang, R.; Duan, C. Chem.Commun. 2016, 52, 9628.
[8] Li, J.; Chen, J.; Jiao, W.; Wang, G.; Li, Y.; Cheng, X.; Li, G. J.Org.Chem. 2016, 81, 9992.
[9] For some examples of radical annulation of α-cyclopropylstyrene:(a) Zhang, F.; Min, Q.-Q.; Zhang, X. Synthesis 2015, 47, 2912.
(b) Prieto, A.; Melot, R.; Bouyssi, D.; Monteiro, N. ACS Catal. 2016, 6, 1093.
(c) Prieto, A.; Melot, R.; Bouyssi, D.; Monteiro, N. Angew.Chem., Int.Ed. 2016, 55, 1885.
[10] (a) Feng, Z.; Xiao, Y.-L.; Zhang, X. Org.Chem.Front. 2016, 3, 466.
(b) Ke, M.; Song, Q. Adv.Synth.Catal. 2017, 359, 384.
(c) Nie, X.; Cheng, C.; Zhu, G. Angew.Chem., Int.Ed. 2017, 56, 1898.
[11] (a) Kimura, T.; Fujita, M.; Sohmiya, H.; Ando, T. J.Org. Chem. 1998, 63, 6719.
(b) Freeman, D. B.; Furst, L.; Condie, A. G.; Stephenson, C. R. J. Org.Lett. 2012, 14, 94.
[12] Cismesia, M. A.; Yoon, T. P. Chem.Sci. 2015, 6, 5426.
[13] (a) Hammond, G. S.; Saltiel, J. J.Am.Chem.Soc. 1962, 84, 4983.
(b) Saltiel, J.; Hammond, G. S. J.Am.Chem.Soc. 1963, 85, 2515.
(c) Hammond, G. S.; Saltiel, J.; Lamola, A. A.; Turro, N. J.; Bradshaw, J. S.; Cowan, D. O.; Counsell, R. C.; Vogt, V.; Dalton, C. J.Am.Chem.Soc. 1964, 86, 3197.
(d) Tatsuo, A.; Hirochika, S.; Katsumi, T. Chem.Lett. 1980, 9, 261.
(e) Arai, T.; Sakuragi, H.; Tokumaru, K. Bull.Chem.Soc.Jpn. 1982, 55, 2204.
(f) Sakaki, S.; Okitaka, I.; Ohkubo, K. Inorg.Chem. 1984, 23, 198.
(g) Osawa, M.; Hoshino, M.; Wakatsuki, Y. Angew.Chem., Int. Ed. 2001, 40, 3472.
[14] (a) Singh, K.; Staig, S. J.; Weaver, J. D. J.Am.Chem. Soc. 2014, 136, 5275.
(b) Singh, A.; Fennell, C. J.; Weaver, J. D. Chem.Sci. 2016, 7, 6796.
[15] (a) Fabry, D. C.; Ronge, M. A.; Rueping, M. Chem.-Eur. J. 2015, 21, 5350. For selected examples involving energy transfer, see:
(b) Chen, Y.; Kamlet, A. S.; Steinman, J. B.; Liu, D. R. Nat.Chem. 2011, 3, 146.
(c) Lu, Z.; Yoon, T. P. Angew.Chem., Int.Ed. 2012, 51, 10329.
(d) Zou, Y.-Q.; Duan, S.-W.; Meng, X.-G.; Hu, X.-Q.; Gao, S.; Chen, J.-R.; Xiao, W.-J. Tetrahedron 2012, 68, 6914.
(e) Alonso, R.; Bach, T. Angew.Chem., Int.Ed. 2014, 53, 4368.
(f) Farney, E. P.; Yoon, T. P. Angew.Chem., Int.Ed. 2014, 53, 793.
(g) Kumarasamy, E.; Raghunathan, R.; Jockusch, S.; Ugrinov, A.; Sivaguru, J. J. Am.Chem.Soc. 2014, 136, 8729.
[16] Metternich, J. B.; Gilmour, R. J.Am.Chem.Soc. 2015, 137, 11254.
[17] Alexander, J.; Renyer, M. L.; Veerapanane, H. Synth.Commun. 1995, 25, 3875.
[18] Jiang, G.-J.; Fu, X-F.; Li, Q.; Yu, Z.-X. Org.Lett. 2012, 14, 692.
[19] Li, J.-Q.; Liu, J.-G.; Krajangsri, S.; Chumnanvej, N.; Singh, T.; Andersson, P. G. ACS Catal. 2016, 6, 8342.
[20] Abascal, N. C.; Lichtor, P. A.; Giuliano, M. W.; Miller, S. J. Chem. Sci. 2014, 5, 45041.
[21] Miller, D. J.; Yu, F.; Young, N. J.; Allemann, R. K. Org.Biomol. Chem. 2007, 5, 3287.
[22] Ren, K.; Hu, B.; Zhao, M.-M.; Tu, Y.-H.; Xie, X.-M.; Zhang, Z.-G. J. Org.Chem. 2014, 79, 2170.
[23] Skapos, H.; Osipov, S. N.; Vorob'eva, D. V.; Odinets, I. L.; Lork, E.; Roeschenthaler, G. Org.Biomol.Chem. 2007, 5, 2361.
/
| 〈 |
|
〉 |