

二萘并呋喃衍生物的合成及其抗肿瘤活性研究
收稿日期: 2017-12-10
修回日期: 2018-01-22
网络出版日期: 2018-02-06
基金资助
黑龙江省青年科学基金(No.QC2014C091)、黑龙江省普通本科高等学校青年创新人才培养计划(No.324015507)、黑龙江省博士后科研启动金(No.LBH-Q16187)资助项目.
Synthesis and Cytotoxicity of Dinaphtho [2, 1-b: 1', 2'-d]furan Derivatives
Received date: 2017-12-10
Revised date: 2018-01-22
Online published: 2018-02-06
Supported by
Project supported by the Youth Science Fund of Heilongjiang Province (No. QC2014C091), the University Nursing Program for Young Scholars with Creative Talents in Heilongjiang Province (No. 324015507) and the Heilongjiang Postdoctoral Scientific Research Developmental Fund (No. LBH-Q16187).
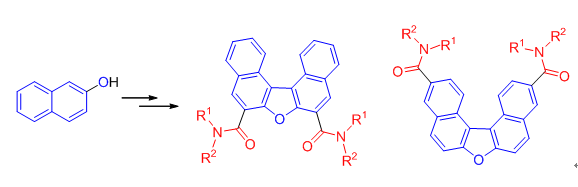
为了研究二萘并呋喃衍生物的构效关系,设计合成了两个系列的二萘[2,1-b:1',2'-d]呋喃衍生物,通过1H NMR、13C NMR、HRMS和IR数据对所有目标化合物进行了结构鉴定.通过噻唑蓝(MTT)法测试了目标化合物的体外抗肿瘤活性,大多数化合物对人肝癌细胞(HepG2和SMMC-7721)、人宫颈癌细胞(HeLa细胞)和急性早幼粒细胞白血病细胞(NB4细胞)显示了较强的抗肿瘤活性.其中N3,N11-二羟基二萘并[2,1-b:1',2'-d]呋喃-3,11-二甲酰胺(13k)对SMMC-7721细胞显示了很强抑制作用,其半数抑制浓度为0.57 μmol·L-1,远低于阳性对照药5-氟脲嘧定的20.21 μmol·L-1.
宋永彬 , 李丹 , 杨异卉 , 纪红蕊 , 刘波 . 二萘并呋喃衍生物的合成及其抗肿瘤活性研究[J]. 有机化学, 2018 , 38(6) : 1516 -1524 . DOI: 10.6023/cjoc201712018
In order to study the structure-activity relationships of dinaphthofuran derivatives, two series of dinaphtho[2,1-b:1', 2'-d]furan derivatives were synthesized. The structures of all compounds were identified by 1H NMR, 13C NMR, HRMS and IR spectra. The in vitro antitumor activity of the synthesized derivatives was tested by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. Most of them exhibited strong inhibitory activity on human hepatocellular carcinoma cell lines (HepG2 and SMMC-7721 cells), uterine cervix cancer Hela cells and acute promyelocytic leukemia NB4 cells. Compound 13k exhibited significant inhibitory activity against SMMC-7721 cells with IC50 vaue of 0.57 μmol·L-1, much lower than 20.21 μmol·L-1 of the positive control 5-Fu.

Key words: synthesis design; cytotoxicity; dinaphthofuran; NMR spectroscopy
[1] Guevel, R. L.; Oger, F.; Lecorgne, A.; Dudasova, Z.; Chevance, S.; Bondon, A.; Barath, P.; Simonneaux, G.; Salbert, G. Bioorg. Med. Chem. 2009, 17, 7021
[2] Ishiguro, K.; Ohira, Y.; Oku, H. J. Nat. Prod. 1998, 61, 1126.
[3] Kotoku, N.; Higashimoto, K.; Kurioka, M.; Arai, M.; Fukuda, A.; Sumii, Y.; Sowa, Y.; Sakai, T.; Kobayashi, M. Bioorg. Med. Chem. Lett. 2014, 24, 3389.
[4] El-Hady, S.; Bukuru, J.; Kesteleyn, B.; Van Puyvelde, L.; Van, T. N.; De Kimpe, N. J. Nat. Prod. 2002, 65, 1377.
[5] Abd El-Wahab, A. H. F.; Al-Fifi, Z. I. A.; Bedair, A. H.; Ali, F. M.; Halawa, A. H. A.; El-Agrody, A. M. Molecules 2011, 16, 307.
[6] Ribeiro-Rodrigues, R.; dos Santos, W. G.; Oliveira, A. B.; Snieckus, V.; Zani, C. L.; Romanha, A. J. Bioorg. Med. Chem. Lett. 1995, 5, 1509.
[7] Bedair, A. H.; Abd El-Wahab, A. H. F.; El-Agrody, A. M.; Ali, F. M.; Halawa, A. H.; El-Sherbiny, G. M. J. Serb. Chem. Soc. 2006, 71, 459.
[8] Stipanovic, R. D.; Bell, A. A.; Howell, C. R. Phytochemistry 1975, 14, 1809.
[9] Doe, M.; Shibue, T.; Haraguchi, H.; Morimoto, Y. Org. Lett. 2005, 7, 1765.
[10] Quillardet, P.; Michel, V.; Arrault, X.; Hofnung, M.; Touati, E. Mutat. Res. 2000, 470, 177.
[11] Touati, E.; Phillips, D. H.; Quillardet, P.; Hofnung, M. Mutagenesis 1993, 8, 149.
[12] Srivastava, V.; Negi, A. S.; Kumar, J. K.; Faridi, U.; Sisodia, B. S.; Darokar, M. P.; Luqman, S.; Khanuja, S. P. S. Bioorg. Med. Chem. Lett. 2006, 16, 911.
[13] Itokawa, H.; Ibraheim, Z. Z.; Qiao, Y. F.; Takeya K. Chem. Pharm. Bull. 1993, 41, 1869.
[14] Lumb, J.-P.; Trauner, D. J. Am. Chem. Soc. 2005, 127, 2870.
[15] Lee, K.-H.; Huang, B.-R. Eur. J. Med. Chem. 2002, 37, 333.
[16] Song, Y. B.; Yang, Y. H.; You, J.; Liu B, Wu, L. J.; Hou, Y. L.; Wang, W. J.; Zhu, J. X. Chem. Pharm. Bull. 2013, 61, 167.
[17] Song, Y. B.; Yang, Y. H.; Wu, L. J.; Dong, N. W.; Gao, S.; Ji, H. R.; Du, X.; Liu, B.; Chen, G. Y. Molecues 2017, 22, 517.
[18] Villemin, D.; Sauvaget, F. Synlett 1994, 6, 435.
[19] Shuklov, I. A.; Dubrovina, N. V.; Jiao, H.; Spannenberg, A.; Börner, A. Eur. J. Org. Chem. 2010, 2010, 1669.
[20] Vilches-Herrera, M.; Miranda-Sepúlveda, J.; Rebolledo-Fuentes, M.; Fierro, A.; Lühr, S.; Iturriaga-Vasquez, P.; Cassels, B. K.; Reyes-Parada, M. Bioorg. Med. Chem. 2009, 17, 2452.
[21] Aoyagi, N.; Ogawa, N.; Izumi, T. Tetrahedron Lett. 2006, 47, 4797.
[22] Kong H.; Yang S.; Gao H.; Timmer, A.; Hill, J. P.; Arado, O. D.; Mönig, H.; Huang, X.; Tang, Q.; Ji, Q.; Liu, W.; Fuchs, H. J. Am. Chem. Soc. 2017, 139, 3669.
[23] Cui, Y.; Ngo, H. L.; Lin, W. Inorg. Chem. 2002, 41, 1033.
/
| 〈 |
|
〉 |