

苯并香豆素羧酸衍生物的合成及荧光性能研究
收稿日期: 2017-12-06
修回日期: 2018-01-28
网络出版日期: 2018-02-11
基金资助
国家自然科学基金(Nos.21373132,21542002)、陕西省教育厅科学研究(No.17JS026)和陕西师范大学研究生创新基金(No.2013CXB026)资助项目.
Synthesis and Fluorescent Property of Benzo [c]coumarin Carboxylic Derivatives
Received date: 2017-12-06
Revised date: 2018-01-28
Online published: 2018-02-11
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21373132, 21542002), the Scientific Research Program Funded by Shaanxi Provincial Education Department (No. 17JS026) and the Innovation Funds of Graduate Programs of Shaanxi Normal University (No. 2013CXB026).
合成了具有较高荧光性能的香豆素衍生物:7-羟基-6-氧代-6H-苯并香豆素-8-羧酸(3a)及2,3-苯并-7-羟基-6-氧代-6H-苯并香豆素-8-羧酸(3b),通过单晶X射线衍射对化合物结构进行了表征.3a和3b在乙醇溶液、晶体结构下具有较高的荧光量子产率(ФF).3a晶体结构存在羧酸二聚体,较强的分子间作用力使其荧光量子效率可达0.32,组成二聚体单元的所有原子处在同一平面内,3a的分子刚性较强.二聚体单元间则通过C—H…O分子间氢键以边对面的堆积方式相连.
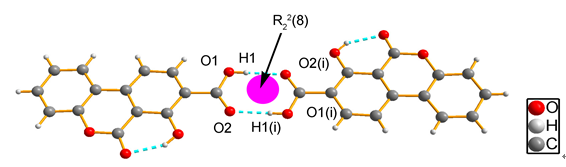
关键词: 苯并香豆素羧酸衍生物; 晶体结构; 二聚体; 荧光性能
史娟 , 张尊听 . 苯并香豆素羧酸衍生物的合成及荧光性能研究[J]. 有机化学, 2018 , 38(6) : 1462 -1468 . DOI: 10.6023/cjoc201712011
Two fluorescent compounds, 7-hydroxy-6-oxo-6H-benzo[c]chromene-8-carboxylic acid (3a) and 2,3-benzo[f]-7-hydroxy-6-oxo-6H-benzo[c]chromene-8-carboxylic acid (3b) have been synthesized and characterized by the method of X-ray single crystal diffraction. 3a and 3b show high fluorescence quantum efficiency in both ethanol solution and solid state. In the crystal-state, dimerization of carboxylic group and intermolecular interaction lead to 3a owned a high fluorescence quantum efficiency (ФF=0.32). The dimers existing in 3a crystal lattice, are composed of carboxylic acid groups and have strong intermolecular interactions (bond lengths are 0.1870 nm, bond angles are 166.8°). All the atoms in dimer are nearly coplanar, showing extremely strong plane rigidity. The dimers are connected by C-H…O intermolecular hydrogen bonds, and the edge-to-face stackings exist in neighbor dimers.

[1] Hu, B. B.; Lu, P.; Wang, Y. G. New J. Chem. 2013, 37, 1645.
[2] Yu, C.; Zhang, J.; Chen, L.; Li, J.; Liu, P.; Wang, W.; Yan, B. Talanta 2011, 85, 1627.
[3] Lee, E. Y.; Jang, S. Y.; Suh, M. P. J. Am. Chem. Soc. 2005, 127, 6374.
[4] Hisaki, I.; Kometani, E.; Shigemitsu, H.; Saeki, A.; Seki, S.; Tohnai, N.; Miyata, M. Cryst. Growth Des. 2011, 11, 5488.
[5] Seth, D. K.; Sakar, D.; Kar, T. CrystEngComm 2011, 13, 4528.
[6] Zhou, T. L.; Jia, T.; Zhao, S. S.; Guo, J. H.; Zhang, H. Y.; Wang, Y. Cryst. Growth Des. 2012, 12, 179.
[7] Ito, H.; Iguchi, A.; Hatano, T. J. Agric. Food Chem. 2008, 56, 393.
[8] Bialonska, D.; Kasimsetty, S. G.; Khan, S. I.; Ferreira, D. J. Agric. Food Chem. 2009, 57, 10181.
[9] Schmidt, J. M.; Tremblay, G. B.; Page, M.; Mercure, J.; Feher, M.; Dunn-Dufault, R.; Peter, M. G.; Redden, P. R. J. Med. Chem. 2003, 46, 1289.
[10] Pandey, J.; Jha, A. K.; Hajela, K. Bioorg. Med. Chem. 2004, 12, 2239.
[11] Appel, B.; Saleh, N. N. R.; Langer, P. Chem.-Eur. J. 2006, 12, 1221.
[12] Nguyen, V. T. H.; Langer, P. Tetrahedron Lett. 2005, 46, 1013.
[13] Ullah, E.; Appel, B.; Fischer, C.; Langer, P. Tetrahedron 2006, 62, 9694.
[14] Nikolov, P.; Petkov, I.; Marko, P. Z. Naturforsch. 2000, 55a, 741.
[15] Chen, C. Y.; Hu, J. S.; Chai, F. F.; Yun, X. K.; Zhang, X. M.; Shi, J. J. Chin. J. Struct. Chem. 2014, 33, 395(in Chinese). (陈超越, 胡劲松, 柴飞飞, 谢凯云, 张晓梅, 石建军, 结构化学, 2014, 33, 395.)
[16] Tasior, M.; Kim, D.; Singha, S.; Krzeszewski, M.; Ahn, K. H.; Gryko, D. T. J. Mater. Chem. C 2015, 3, 1421.
[17] Jiao, L.; Wu, Y.; Ding, Y.; Wang, S.; Zhang, P.; Yu, C.; Wei, Y.; Mu, X.; Hao, E. Chem.-Asian J. 2014, 9, 805.
[18] Bernstein, J.; Davis, R. E.; Shimoni, L.; Chang, N. L. Angew. Chem., Int. Ed. 1995, 34, 1555.
[19] Sun, J. B.; Zhang, G. H.; Jia, X. Y.; Xue, P. C.; Jia, J. P.; Lu, R. Acta Chim. Sinica 2016, 74, 165(in Chinese). (孙静波, 张恭贺, 贾小宇, 薛鹏冲, 贾俊辉, 卢然, 化学学报, 2016, 74, 165.)
[20] Feng, Q.; Wang, M.; Dong, B.; Xu, C.; Zhao, J.; Zhang, H. CrystEngComm 2013, 15, 3623.
[21] Cheng, X.; Wang, K.; Huang, S.; Zhang, H. Y.; Zhang, H. Y.; Wang, Y. Angew. Chem., Int. Ed. 2015, 54, 1.
[22] Yu, T. Z.; Zhao, Y. L.; Fan, D. W. J. Mol. Struct. 2006, 1~3, 18.
[23] Sheldrick, G. M. SHELXS-97, Program for X-Ray Crystal Structure Solution, University of Götingen, Germany, 2007.
[24] Sheldrick, G. M. Acta Crystallogr. 2008, A64, 112.
/
| 〈 |
|
〉 |