

无碱条件下的Cu2O催化绿色氧化端基炔偶联
收稿日期: 2017-12-27
修回日期: 2018-01-31
网络出版日期: 2018-02-11
基金资助
浙江省自然科学基金(No.LY17B030011)及嘉兴市科技计划(No.2015AY11014)资助项目.
Cu2O-Catalyzed Green Oxidative Terminal Alkynes Homocoupling without Bases
Received date: 2017-12-27
Revised date: 2018-01-31
Online published: 2018-02-11
Supported by
Project supported by the National Natural Science Foundation of Zhejiang Province (No. LY17B030011) and the Jiaxing Science and Technology Project (No. 2015AY11014).
马楠 , 曾祥华 . 无碱条件下的Cu2O催化绿色氧化端基炔偶联[J]. 有机化学, 2018 , 38(6) : 1556 -1561 . DOI: 10.6023/cjoc201712038
A high efficient method for the synthesis of 1,3-diynes derivatives which employed terminal alkynes as the substrates and copper(I) oxide as the catalyst was developed. This method possessed the character of base-free and mild reaction conditions. The reaction mechanism was also studied. Furthermore, this reaction could be magnified to gram scale and the catalyst of copper(I) oxide could be recycled.
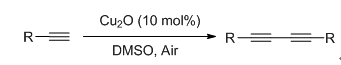
Key words: copper (I) oxide; homocoupling; terminal alkynes; base-free
[1] (a) Lerch, M. L.; Harper, M. K.; Faulkner, D. J. J. Nat.Prod. 2003, 66, 667.
(b) Lechner, D.; Stavri, M.; Oluwatuyi, M.; Perda-Miranda, R.; Gibbons, S. Phytochemistry 2004, 65, 331.
(c) Constable, C. P.; Towers, G. H. N. Planta Med. 1989, 55, 35.
(d) Zhou, Y. Z.; Ma, H. Y.; Chen, H.; Qiao, L.; Yao, Y.; Cao, J. Q.; Pei, Y. H. Chem.Pharm.Bull. 2006, 54, 1455.
(e) Ladika, M.; Fisk, T. E.; Wu, W. W.; Jons, S. D. J.Am.Chem. Soc. 1994, 116, 12093.
(f) Mayer, S. F.; Steinreiber, A.; Orru, R. V. A.; Faber, K. J.Org. Chem. 2002, 67, 9115.
(g) Zeni, G.; Panatieri, R. B.; Lissner, E.; Menezes, P. H.; Braga, A. L.; Stefani, H. A. Org.Lett. 2001, 3, 819.
(h) Stüts, A. Angew.Chem., Int.Ed.Engl. 1987, 26, 320.
[2] (a) Gholami, M.; Tykwinski, R. R. Chem.Rev. 2006, 106, 4997.
(b) Baxter, P. N. W.; Dali-Youcef, R. J.Org.Chem. 2005, 70, 4935.
(c) Marsden, J. A.; Haley, M. M. J.Org.Chem. 2005, 70, 10213.
[3] (a) Cataldo, F. In Polyynes:Synthesis Properties, and Applications, CRC Press/Taylor & Francis, Boca Raton, Florida, 2005.
(b) Diederich, F.; Stang, P. J.; Tykwinski, R. R. Acetylene Chemistry:Chemistry, Biology and Material Science, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2005.
[4] Crowley, J. D.; Goldup, S. M.; Lee, A. L.; Leigh, D. A.; McBurney, R. T. Chem.Soc.Rev. 2009, 38, 1530.
[5] Glaser, C. Ber.Dtsch.Chem.Ges. 1869, 2, 422.
[6] Chen, L.; Lemma, B. E.; Rich, J. S.; Mack, J. Green Chem. 2014, 16, 1101.
[7] Mo, G.; Tian, Z.; Li, J.; Wen, G.; Yang, X. Appl. Organomet.Chem. 2015, 29, 231.
[8] (a) Leyva-Perez, A.; Domenech, A.; Al-Resayes, S. I.; Corma, A. ACS Catal. 2012, 2, 121.
(b) Peng, H.; Xi, Y.; Ronaghi, N.; Dong, B.; Akhmedov, G. N.; Shi, X. J. Am.Chem.Soc. 2014, 136, 13174.
[9] (a) Fan, X.; Li, N.; Shen, T.; Cui, X.-M.; Lv, H.; Zhu, H.-B.; Guan, Y.-H. Tetrahedron 2014, 70, 256.
(b) Yin, K.; Li, C.-J.; Li, J.; Jia, X.-S. Appl.Organomet.Chem. 2011, 25, 16.
(c) Navale, B. S.; Bhat, R. G. RSC Adv. 2013, 3, 5220.
(d) Zhang, S.; Liu, X.; Wang, T. Adv.Synth.Catal. 2011, 353, 1463.
(e) Jia, X.; Yin, K.; Li, C.; Li, J.; Bian, H. Green Chem. 2011, 13, 2175.
(f) Balaraman, K.; Kesavan, V. Synthesis 2010, 3461.
(g) Kusuda, A.; Xu, X.-F.; Wang, X.; Tokunaga, E.; Shibata, N. Green Chem. 2011, 13, 843.
(h) Adimurthy, S.; Malakar, C. C.; Beifuss, U. J. Org.Chem. 2009, 74, 5648.
(i) Yin, K.; Li, C.; Li, J.; Jia, X. Green Chem. 2011, 13, 591.
(j) Wang, D.; Li, J.; Li, N.; Gao, T.; Hou, S.; Chen, B. Green Chem. 2010, 12, 45.
(k) Kabalka, G. W.; Wang, L.; Pagni, R. M. Synlett 2001, 108.
(l) Li, Y.-N.; Wang, J.-L.; He, L.-N. Tetrahedron Lett. 2011, 52, 3485.
(m) Sagadevan, A.; Charpe, V. P.; Hwang, K. C. Catal. Sci.Technol. 2016, 6, 7688.
(n)Sagadevan, A.; Lyu, P.-C.; Hwang, K. C. Green Chem. 2016, 18, 4526.
(o) Oishi, T.; Katayama, T.; Yamaguchi, K.; Mizuno, N. Chem.Eur.J. 2009, 15, 7539.
(p) Oishi, T.; Yamaguchi, K.; Mizuno, N. ACS Catal. 2011, 1, 1351.
(q) Zhu, Y.; Shi, Y. Org. Biomol. Chem. 2013, 11, 7451.
(r) Kamata, K.; Yamaguchi, S.; Kotani, M.; Yamaguchi, K.; Mizuno, N. Angew.Chem., Int.Ed. 2008, 47, 2407.
[10] Allen, S. E.; Walvoord, R. R.; Padilla-Salinas, R.; Kozlowski, M. C. Chem.Rev. 2013, 113, 6234.
[11] Yin, W.; He, C.; Chen, M.; Zhang, H.; Lei, A. Org.Lett. 2009, 11, 709.
[12] Ye, R.; Zhukhovitskiy, A. V.; Deraedt, C. V.; Toste, F. D.; Somorjai, G. A. Acc.Chem.Res. 2017, 50, 1894.
[13] Tang, B.-X.; Fang, X.-N.; Kuang, R.-Y.; Wu, J.-H.; Chen, Q.; Hu, S.-J.; Liu, Y.-L. Appl.Organomet.Chem. 2016, 30, 943.
[14] Su, L.; Dong, J.; Liu, H.; Sun, M.; Qiu, R.; Zhou, Y.; Yin, S.-F. J. Am.Chem.Soc. 2016, 138, 12348.
[15] Xu, H.; Wu, K.; Tian, J.; Zhu, L.; Yao, X. Green Chem. 2018, 20, 793.
[16] Theunissen, C.; Evano, G. Org.Lett. 2014, 16, 4488.
[17] Chinchilla, R.; Najera, C. Chem.Soc.Rev. 2011, 40, 5084.
/
| 〈 |
|
〉 |