

三叔丁基膦亚胺苯氧钛配合物及其乙烯聚合性能
收稿日期: 2018-01-04
修回日期: 2018-02-09
网络出版日期: 2018-03-08
基金资助
中国石油化工股份有限公司技术开发项目(No.214002)资助项目.
Synthesis of Tritertbutylphosphinimine Phenoxy Titanium Complexes and Their Catalytic Performance to Ethylene Polymerization
Received date: 2018-01-04
Revised date: 2018-02-09
Online published: 2018-03-08
Supported by
Project supported by the Technology Development Project of China Petroleum & Chemical Corporation (Sinopec) (No. 214002).
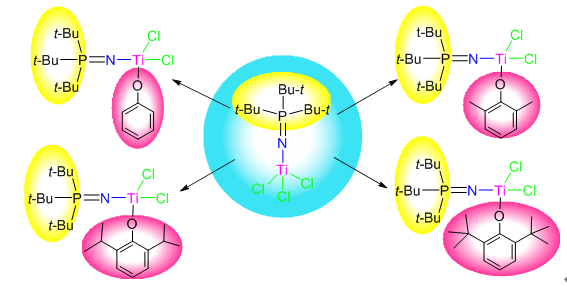
四种不同基团取代的苯酚锂盐分别与三叔丁基膦亚胺三氯化钛进行反应,制得相应三叔丁基膦亚胺苯氧钛配合物(t-Bu3) PNTi(OAr) Cl2[OAr=O-C6H5(4a),O-2,6-Me2C6H3(4b),O-2,6-i-Pr2C6H3(4c)和O-2,6-t-Bu2C6H3(4d)],产物均借助1H NMR,13C NMR,31P NMR及元素分析进行了结构表征,并利用X射线单晶衍射确定了配合物三叔丁基膦亚胺三氯化钛(3),4b和4d的分子结构.在助催化剂甲基铝氧烷(MAO)作用下,4a~4d对乙烯聚合均表现出高催化活性,并随配合物空间位阻增加而升高.4c热稳定性好,通过控制聚合反应条件,由此可以得到不同分子量及分子量分布的聚乙烯产物.
王铁石 , 陈建军 , 叶霖 , 张爱英 , 冯增国 . 三叔丁基膦亚胺苯氧钛配合物及其乙烯聚合性能[J]. 有机化学, 2018 , 38(6) : 1544 -1548 . DOI: 10.6023/cjoc201801004
Tritertbutylphosphinimine phenoxy titanium complexes (t-Bu3)PNTi(OAr)Cl2 [Ar=C6H5(4a), 2,6-Me2C6H3 (4b), 2,6-i-Pr2C6H3 (4c) and 2,6-t-Bu2C6H3 (4d)] were synthesized via the reaction of corresponding substituted phenol lithium salts with tritertbutylphosphinimine trichloride titanium (3). The compounds were characterized by means of 1H NMR, 13C NMR, 31P NMR spectroscopic and elemental analyses, and the molecular structures of 3, 4b and 4d were further confirmed by single-crystal X-ray diffraction analysis. When activated with methylaluminoxane (MAO), 4a~4d displayed not only high catalytic activities, but also increasing performances on ethylene polymerization with increasing the sterical hindrance of substituents. Furthermore, 4c depicted a good thermal stability, with which the polyethylene products of different molecular weights and molecular weight distributions can be obtained by tuning the polymerization conditions.

[1] Britovsek, G. J. P.; Gibson, V. C.; Wass, D. F. Angew. Chem. 1999, 111, 448.
[2] Valente, A.; Mortreux, A.; Visseaux, M.; Zinck, P. Chem. Rev. 2013, 113, 3836.
[3] Stehling, U.; Diebold, J.; Kirsten, R.; Roll, W.; Brintzinger, H.-H.; Jungling, S.; Mulhaupt, R.; Langhauser, F. Organometallics 1994, 13, 964.
[4] Resconi, L.; Balboni, D.; Baruzzi, G.; Fiori, C.; Guidotti, S. Organometallics 2000, 19, 420.
[5] Ewen, J. A.; Jones, R. L.; Elder, M. J.; Rheingold, A. L.; Liable-Sands, L. M. J. Am. Chem. Soc. 1998, 120, 10786.
[6] Chen, J. J.; Wang, T. S.; Tang, Z. W.; Xu, Y. B.; Xu, L.; Cao, M. S.; Feng, Z, G. Acta Polym. Sin. 2017, 1294(in Chinese). (陈建军, 王铁石, 唐正伟, 徐一兵, 徐林, 曹茂盛, 冯增国, 高分子学报, 2017, 1294.)
[7] Yamasaki, H.; Kimura, K.; Nakano, M.; Ushioda, T. Chem. Lett. 1999, 1311.
[8] Su, B. Y.; Jia, P. Y.; Wang, Y. Z.; Li, Y. N.; Huang, H.; Li, Q. D. Chin. J. Org. Chem. 2016, 36, 2344(in Chinese). (苏碧云, 郏佩瑜, 王彦昭, 李亚宁, 黄鹤, 李谦定, 有机化学, 2016, 36, 2344.)
[9] Kang, X. H.; Zhou, G. L.; Wang, X. B.; Qu, J. P.; Hou, Z. M.; Luo, Y. Organometallics 2016, 35, 913.
[10] Xu, S.; Liang, C. C.; Lv, Z. W.; Zhu, Y. L.; Zhang, C.; Mi, P. K. Chin. J. Org. Chem. 2017, 37, 1284(in Chinese). (许胜, 梁春超, 吕中文, 朱玉玲, 张翠, 米普科, 有机化学, 2017, 37, 1284.)
[11] Qi, C. H.; Zhang, S. B. Appl. Organomet. Chem. 2006, 20, 70.
[12] Liu, K.; Wu, Q.; Gao, W.; Mu, Y.; Ye, L. Eur. J. Inorg. Chem. 2011, 12, 1901.
[13] Liu, Q. Y.; Gao, R.; Hou, J. X.; Sun, W. H. Chin. J. Org. Chem. 2013, 33, 808(in Chinese). (刘清云, 高榕, 侯俊先, 孙文华, 有机化学, 2013, 33, 808.)
[14] Nomura, K.; Liu, J. Dalton Trans. 2011, 7666.
[15] Kakinuki, K.; Fujiki, M.; Nomura, K. Macromolecules 2009, 42, 4585.
[16] Nomura, K.; Pengoubol, S.; Apisuk, W. RSC Adv. 2016, 6, 16203.
[17] Hu, W. Q.; Sun, X. L.; Wang, C.; Gao, Y.; Tang, Y.; Shi, L. P.; Xia, W.; Sun, J.; Dai, H. L.; Li, X. Q.; Yao, X. L.; Wang, X. R. Organometallics 2004, 23, 1684.
[18] Stephan, D. W.; Guerin, F.; Spence, R. E. v. H.; Koch, L.; Gao, X. L.; Brown, S. J.; Swabey, J. W.; Wang, Q. Y.; Xu, W.; Zoricak, P.; Harrison, D. G. Organometallics 1999, 18, 2046.
[19] Stephan, D. W.; Stewart, J. C.; Guerin, F.; Spence, R. E. v. H.; Xu, W.; Harrison, D. G. Organometallics 1999, 18, 1116.
[20] Stephan, D. W. Organometallics 2005, 24, 2548.
[21] Yue, N.; Hollink, E.; Guerin, F.; Stephan, D. W. Organometallics 2001, 20, 4424.
[22] Hollink, E.; Stewart, J. C.; Wei, P. R.; Stephan, D. W. Dalton Trans. 2003, 3968.
[23] Hollink, E.; Stewart, J. C.; Wei, P. R.; Stephan, D. W. Can. J. Chem. 2004, 82, 1304.
[24] Yuan, S. F.; Wang, L. J.; Zhang, Q. Y.; Sun, W. H. Prog. Chem. 2017, 29, 1462(in Chinese). (袁世芳, 王丽静, 张秋月, 孙文华, 化学进展, 2017, 29, 1462.)
[25] Fenwick, A. E.; Phomphrai, K.; Thorn, M. G.; Vilardo, J. S.; Trefun, C. A.; Hanna, B.; Fanwick, P. E.; Rothwell, I. P. Organometallics 2004, 23, 2146.
[26] Phomphrai, K.; Fenwick, A. E.; Sharma, S.; Fanwick, P. E.; Caruthers, J. M.; Delgass, W. N.; Abu-Omar, M. M.; Rothwell, I. P. Organometallics 2006, 25, 214.
[27] Nomura, K.; Naga, N.; Miki, M.; Yanagi, K. Macromolecules 1998, 31, 7588.
[28] Yan, Q.; Yang, W. H.; Chen, L. Q.; Wang, L.; Redshaw, C.; Sun, W. H. J. Organomet. Chem. 2014, 753, 34.
[29] Lv, Y.; Wang, S. C.; Wu, B.; Zheng, Q. T.; Han, G. Q.; Sun, H. D. Acta Acad. Med. Sin. 1999, 21, 1(in Chinese). (吕扬, 王树春, 吴斌, 郑启泰, 韩桂秋, 孙汉董, 中国医学科学院学报, 1999, 21, 1.)
/
| 〈 |
|
〉 |