

一类芳香酰胺螺旋折叠聚合物的设计与合成
收稿日期: 2017-12-29
修回日期: 2018-02-14
网络出版日期: 2018-03-16
基金资助
国家自然科学基金(No.20772123)资助项目.
Design and Synthesis of a Class of Aromatic Polyamide Foldamer
Received date: 2017-12-29
Revised date: 2018-02-14
Online published: 2018-03-16
Supported by
Project supported by the National Natural Science Foundation of China (No. 20772123).
王恒 , 张宇坤 , 邹胜 , 曹金鑫 , 黄晴菲 , 王启卫 , 朱槿 . 一类芳香酰胺螺旋折叠聚合物的设计与合成[J]. 有机化学, 2018 , 38(8) : 2060 -2066 . DOI: 10.6023/cjoc201712043
Long aromatic polymer chains which based on 3,5-diaminobenzoic acid derivatives and 4,6-dihydroxyisophthalic acid derivatives are designed and synthesized. The results of solubility, NMR, UV-Vis spectrum and circular dichroism (CD) experments indicate that the polymer adopts a hollow helical conformation that is stablized by intramolecular H-bonding interaction between side chains.
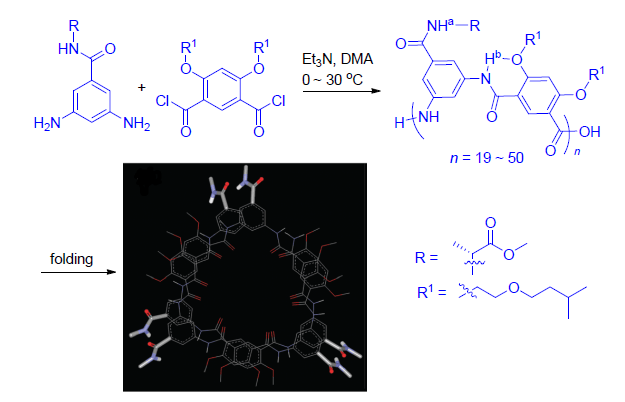
Key words: aromatic polyamide; foldamer; helice
[1] Gellman, S. H. Acc. Chem. Res. 1998, 31, 173.
[2] David, J. H.; Matthew J. M.; Ryan, B. P.; Thomas, S. H.; Moore, J. S. Chem. Rev. 2001, 101, 3893.
[3] Gong, B. Chem.-Eur. J. 2001, 7, 4336.
[4] Huc, I. Eur. J. Org. Chem. 2004, 17.
[5] Li, Z. T.; Hou, J. L.; Li, C.; Yi, H.-P. Chem.-Asian J. 2006, 1, 766.
[6] Goodman, C. M.; Choi, S.; Shandler, S.; Degrado, W. F. Nat. Chem. Biol. 2007, 3, 252.
[7] Guichard, G.; Huc, I. Chem. Commun. 2011, 47, 5933.
[8] Appella, D. H.; Christianson, L. A.; Karle, I. L.; Powell, D. R.; Gellman, S. H. J. Am. Chem. Soc. 1996, 118, 13071.
[9] Yang, D.; Li, W.; Qu, J.; Luo, S.-W.; Wu, Y. D. J. Am. Chem. Soc. 2003, 125, 13018.
[10] Nelson, J. C.; Saven, J. G.; Moore, J. S.; Wolynes, P G. Science 1997, 277, 1793.
[11] Seebach, D.; Overh, M.; Kiihnle, F. N. M.; Martinoni, B.; Oberer, L.; Hommel, U.; Widmer, H. Helv. Chim. Acta 1996, 79, 913.
[12] Hamuro, Y.; Geib, S. J.; Hamilton, A. D. J. Am. Chem. Soc. 1997, 119, 10587.
[13] Zhu, J.; Parra, R. N. D.; Zeng, H.; Skrzypczak-Jankun, E.; Zeng, X C.; Gong, B. J. Am. Chem. Soc, 2000, 122, 4219.
[14] Jiang, H.; LéGer, J.-M.; Huc, I. J. Am. Chem. Soc. 2003, 125, 3448.
[15] Hou, J. L.; Shao, X. B.; Chen, G. J.; Zhou, Y. X.; Jiang, X. K.; Li, Z. T. J. Am. Chem. Soc. 2004, 126, 12386.
[16] Wang, Y.; Wang, H.; Zhang, D.-W.; Li, Z.-T. Chin. J. Org. Chem. 2016, 36, 1580(in Chinese). (汪奕, 王辉, 张丹维, 黎占亭, 有机化学, 2016, 36, 1580)
[17] Sun, G.; Nie, C.; Zhao, X.; Li, Z. Chin. J. Org. Chem. 2017, 37, 1757(in Chinese). (孙广军, 聂承斌, 赵新, 黎占亭, 有机化学, 2017, 37, 1757.)
[18] Yang, L.; Zhao, W.; Che, Y. K.; Wang, Y.; Jiang, H. Chin. Chem. Lett. 2017, 28, 1659.
[19] Van Gorp, J J.; Vekemans, J. A. J. M.; Meijer, E. W. Chem. Commun. 2004, 60.
[20] Sinkeldam, R. W.; Van Houtem, M. H. C. J.; Pieterse, K.; Vekemans, J. A. J. M.; Meijer, E. W. Chem.-Eur. J. 2006, 12, 6129.
[21] Lu, Y. X.; Shi, Z. M.; Li, Z. T.; Guan, Z. Chem. Commun. 2010, 46, 9019.
[22] Cao, J.; Kline, M.; Chen, Z.; Luan, B.; Lv, M.; Zhang, W.; Lian, C.; Wang, Q.; Huang, Q.; Wei, X.; Deng, J.; Zhu, J.; Gong, B. Chem. Commun. 2012, 48, 11112.
[23] Zhang, Y.-C.; Chen, L.; Wang, H.; Zhou, Y.-M.; Zhang, D.-W.; Li, Z.-T. Chin. Chem. Lett. 2016, 27, 817.
[24] Zhang, P.; Wang, Z.; Zhang, L.; Wang, H.; Zhang, D.; Hou, J.; Li, Z. Chin. J. Chem. 2016, 34, 678.
[25] Gong, B. Acc. Chem. Res. 2008, 41, 1376.
[26] Parra, R. D.; Zeng, H.; Zhu, J.; Zheng, C.; Zeng, X. C.; Gong, B. Chem.-Eur. J. 2001, 7, 4352.
[27] Feng, W.; Yamato, K.; Yang, L.; Ferguson, J. S.; Zhong, L.; Zou, S.; Yuan, L.; Zeng, X. C.; Gong, B. J. Am. Chem. Soc. 2009, 131, 2629.
[28] Aimin, Z.; Joseph S, F.; Kazuhiro, Y.; Chong, Z.; Gong, B. Org. Lett. 2006, 8, 5117.
[29] Wei, X.; Zhang, G.; Shen, Y.; Zhong, Y.; Liu, R.; Yang, N.; Al-Mkhaizim, F Y.; Kline, M A.; He, L.; Li, M.; Lu, Z. L.; Shao, Z.; Gong, B. J. Am. Chem. Soc. 2016, 138, 2749.
[30] García, J. M.; García, F. C.; Serna, F.; de la Peña, J. L. Prog. Polym. Sci. 2010, 35, 623.
[31] Sakurai, K.; Mizu, M.; Shinkai, S. Biomacromolecules, 2001, 2, 641.
[32] Creighton, T. E. Proteins:Structures and Molecular Properties, 2nd ed., W. H. Freeman, New York, 1993.
[33] Vicente, A. I.; Caio, J. M.; Sardinha, J.; Moiteiro, C.; Delgado, R.; Félix, V. Tetrahedron 2012, 68, 670.
/
| 〈 |
|
〉 |