

新型含取代噁唑结构的氰基丙烯酸酯类化合物的合成与除草活性研究
收稿日期: 2018-02-26
修回日期: 2018-03-09
网络出版日期: 2018-03-16
基金资助
国家自然科学基金(No.21372135)、江苏省“六大人才高峰”(No.2013-SWYY-013)、南通市科技计划(No.MS22015020)和江苏省大学生创新训练计划(No.201710304048)资助项目.
Synthesis and Herbicidal Activity of Novel Cyanoacrylate Derivatives Containing Substituted Oxazole Moiety
Received date: 2018-02-26
Revised date: 2018-03-09
Online published: 2018-03-16
Supported by
Project supported by the National Natural Science Foundation of China (No. 21372135), the Research Foundation of the Six People Peak of Jiangsu Province (No. 2013-SWYY-013), the Science and Technology Project Fund of Nantong City (No. MS22015020), and the Science and Technology Innovation Foundation for the College Students of Jiangsu Province (No. 201710304048).
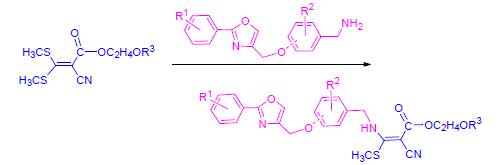
为了从氰基丙烯酸酯类化合物中寻找新的活性物质,采用活性亚结构拼接原理,设计并制备了一系列未见文献报道的新型含取代噁唑结构的氰基丙烯酸酯类衍生物.借助于1H NMR、13C NMR和元素分析等手段对目标化合物的结构进行了确证.初步的生测结果表明,在剂量为1500 g/ha时,大多数目标化合物具有较好的除草活性,9个化合物对芥菜的除草活性可达80%~100%,12个化合物对小藜的除草活性可达80%~100%,2个化合物对看麦娘的除草活性均为70%,一个化合物对酸模、棒头草和早熟禾的抑制率分别为100%,70%和60%;此外,一个化合物在37.5 g/ha剂量下对芥菜和酸模的抑制率均为30%.
石玉军 , 杜显超 , 王祥龙 , 陈庆文 , 李玲 , 戴红 , 徐蔡芹 , 张敬远 , 凌勇 . 新型含取代噁唑结构的氰基丙烯酸酯类化合物的合成与除草活性研究[J]. 有机化学, 2018 , 38(7) : 1772 -1778 . DOI: 10.6023/cjoc201802026
In search of novel cyanoacrylates with potent bioactivity, a series of new cyanoacrylate derivatives containing substituted oxazole moiety were designed and synthesized through the strategy of active substructure combination. They were structurally characterized by 1H NMR, 13C NMR and elemental analysis. Preliminary bioassay data showed that most title compounds possessed good herbicidal activity at the dosage of 1500 g/ha, 9 compounds exhibited herbicidal activity against Brassica juncea with 80%~100%, 12 compounds had herbicidal activity against Chenopodium serotinum L. with 80%~100%, two compounds both displayed 70% herbicidal activity against Alopecurus aequalis Sobol, and one compound showed 100%, 70%, and 60% herbicidal activity against Rumex acetosa L., Polypogon fugax Nees ex Steud and Poa acroleuca Steud, respectively. Additionally, the inhibitory rates of one compound against Brassica juncea and Rumex acetosa L.were both 30% at 37.5 g/ha.

Key words: oxazole; cyanoacrylate; synthesis; herbicidal activity
[1] Liu, H. Y.; Tan, H. F.; Yang, H. Z. Chem. J. Chin. Univ. 2000, 21, 1855 (in Chinese).
(刘华银, 谭惠芬, 杨华铮, 高等学校化学学报, 2000, 21, 1855.)
[2] Song, B. A.; Yang, S.; Zhong, H. M.; Jin, L. H.; Hu, D. Y.; Lin, G. J. Fluorine Chem. 2005, 126, 87.
[3] Ouyang, G. P.; Song, B. A.; Zhang, H. P.; Yang, S.; Jin, L. H.; Li, Q. Z.; Hu, D. Y. Molecules, 2005, 10, 1351.
[4] Liu, Y. X.; Cao, B. L.; Li, Y. H.; Song, H. B.; Huang, R. Q.; Wang, Q. M. J. Agric. Food Chem. 2007, 55, 3011.
[5] Zhong, S. H.; Wang, C. F.; Song, Q. X.; Fan, M. L.; Liu, B. Y.; Wei, D. M.; Liu, J. B. Chin. J. Org. Chem. 2014, 34, 2324 (in Chinese).
(钟世华, 王春凤, 宋青霞, 范明亮, 刘兵玉, 危冬梅, 刘建兵, 有机化学, 2014, 34, 2324.)
[6] Wang, X.; Wang, C. Q.; Fu, C. R.; Zou, X. M. Chin. J. Org. Chem. 2015, 35, 92 (in Chinese).
(王鑫, 王朝强, 傅翠蓉, 邹小毛, 有机化学, 2015, 35, 92.)
[7] Wu, S. S.; Miao, W. K.; Wang, T. T.; Fang, J. X. Chin. J. Org. Chem. 2015, 35, 1484 (in Chinese).
(吴珊珊, 苗文科, 王婷婷, 方建新, 有机化学, 2015, 35, 1484.)
[8] Shi, Y. J.; Li, Y.; Fang, Y.; Chen, J.; Ye, L. Y.; Ge, S. S.; Dai, H. Chin. J. Org. Chem. 2016, 36, 2472 (in Chinese).
(石玉军, 李阳, 方源, 陈佳, 叶林玉, 葛书山, 戴红, 有机化学, 2016, 36, 2472.)
[9] Wang, Q. M.; Sun, H. K.; Cao, H. Y.; Cheng, M. R.; Huang, R. Q. J. Agric. Food Chem. 2003, 51, 5030.
[10] Song, B. A.; Zhang, H. P.; Wang, H.; Yang, S.; Jin, L. H.; Hu, D. Y.; Pang, L. L.; Xue, W. J. Agric. Food Chem. 2005, 53, 7886.
[11] Zhao, Q. Q.; Liu, S. H.; Li, Y. H.; Wang, Q. M. J. Agric. Food Chem. 2009, 57, 2849.
[12] Shi, Y. J.; Fang, Y.; Li, Y.; Chen, J.; Li, G.; Wang, Q. M.; Dai, H. Chem. J. Chin. Univ. 2017, 38, 421 (in Chinese).
(石玉军, 方源, 李阳, 陈佳, 李刚, 汪清民, 戴红, 高等学校化学学报, 2017, 38, 421.)
[13] Wang, M. M.; Zhang, Q. Q.; Yue, K.; Li, Q. S.; Xu, F. B. Chin. J. Org. Chem. 2017, 37, 1774 (in Chinese).
(王梦梦, 张青青, 岳凯, 李庆山, 徐凤波, 有机化学, 2017, 37, 1774.)
[14] Gideens, A. C.; Boshoff, H. I. M.; Franzblau, S. G.; Barry Ⅲ, C. E.; Coppa, B. R. Tetrahedron Lett. 2005, 46, 7355.
[15] Prakash, T. B.; Reddy, G. D.; Padmaja, A.; Padmavathi, V. Eur. J. Med. Chem. 2014, 82, 347.
[16] Lin, J.; Chen, J. W.; Cai, X. Y.; Qiao, X. L.; Huang, L. P.; Wang, D. G.; Wang, Z. J. Agric. Food Chem. 2007, 55, 7626.
[17] Wu, C.; Liang, Z. W.; Xu, Y. Y.; He, W. M.; Xiang, J. N. Chin. Chem. Lett. 2013, 24, 1064.
[18] Zhong, Z. J.; Zhang, D. J.; Peng, Z. G.; Li, Y. H.; Shan, G. Z.; Zuo, L. M.; Wu, L. T.; Li, S. Y.; Gao, R. M.; Li, Z. R. Eur. J. Med. Chem. 2013, 69, 347.
[19] Ohnmacht, S. A.; Ciancimino, C.; Vignaroli, G.; Gunaratnam, M.; Neidle, S. Bioorg. Med. Chem. Lett. 2013, 23, 5351.
[20] Xiao, L. W.; Zhang, G. X.; Jing, X. M.; Zhou, Q. X.; Feng, R. Chin. J. Org. Chem. 2016, 36, 1000 (in Chinese).
(肖立伟, 张光霞, 景学敏, 周秋香, 冯茹, 有机化学, 2016, 36, 1000.)
[21] Wang, S. L.; Shi, Y. J.; He, H. B.; Li, Y.; Li, Y.; Dai, H. Chin. Chem. Lett. 2015, 26, 672.
[22] Dai, H.; Xiao, Y. S.; Li, Z.; Xu, X. Y.; Qian, X. H. Chin. Chem. Lett. 2014, 25, 1014.
[23] Dai, H.; Chen, J.; Hong, Yu.; Yuan, B. Y.; Chen, Y. M.; Shi, Y. J.; Ma, R. Y.; Liang, Z. P.; Shi, J. Chin. J. Org. Chem. 2017, 37, 739 (in Chinese).
(戴红, 陈佳, 洪宇, 袁斌颖, 陈雨蒙, 石玉军, 马瑞媛, 梁志鹏, 石健, 有机化学, 2017, 37, 739.)
[24] Dai, H.; Fang, Y.; Li, Y.; Wang, X. L.; Xiang, X. B.; Ge, S. S.; Shi, Y. J. Chin. J. Org. Chem. 2016, 36, 2973 (in Chinese).
(戴红, 方源, 李扬阳, 王祥龙, 向兴邦, 葛书山, 石玉军, 有机化学, 2016, 36, 2973.)
/
| 〈 |
|
〉 |