

南鹤虱中两个新的倍半萜类化合物
收稿日期: 2018-01-19
修回日期: 2018-02-26
网络出版日期: 2018-04-04
基金资助
国家自然科学基金(No.31560102)资助项目.
Two New Sesquterpenoids from Fructus Carotae
Received date: 2018-01-19
Revised date: 2018-02-26
Online published: 2018-04-04
Supported by
Project supported by the National Natural Science Foundation of China (No. 31560102).
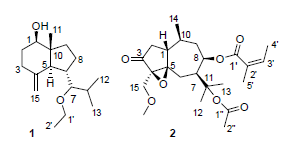
从中药南鹤虱(Fructus carotae)中分离纯化得到7个倍半萜类化合物,分别鉴定为7-ethoxy-4(15)-oppositen-1β-ol(1),11-乙酰氧基-8β-当归酰氧基-15-甲氧基-4α,5α-环氧愈创木烷-3-酮(2),11-乙酰氧基-8β-丙酰氧基-4-愈创木烯-3-酮(3),1-oxo-5α,7αH-eudesma-3-en-15-al(4),1β-hydroxy-4(15),7-eudesmadiene(5),1β-hydroxy-4(15),5E,10(14)-germacra-triene(6),1β-hydroxy-4(15),5-eudesmadiene(7).其中化合物1和2为新的化合物,新化合物的结构进一步通过HR-ESIMS,1D NMR和2D NMR等光谱技术确定.
成蕾 , 刘贵园 , 潘胤池 , 张茂生 , 肖世基 . 南鹤虱中两个新的倍半萜类化合物[J]. 有机化学, 2018 , 38(7) : 1829 -1832 . DOI: 10.6023/cjoc201801027
Seven sesquterpenoids were obtained from the Chinese traditional medicine, Fructus carotae, and their structures were identified as 7-ethoxy-4(15)-oppositen-1β-ol (1), 11-acetoxy-8β-angeloyloxy-15-methoxy-4α,5α-epoxyarbutane-3-one (2), 11-acetoxy-8β-propionyl-4-guaien-3-one (3), 1-oxo-5α,7αH-eudesma-3-en-15-al (4), 1β-hydroxy-4(15),7-eudesmadiene (5), 1β-hydroxy-4(15),5E,10(14)-germacratriene (6) and 1β-hydroxy-4(15),5-eudesmadiene (7). Compounds 1 and 2 were new sesquterpenoid compounds, and their structures were determined by the analysis of HR-ESIMS, 1D NMR and 2D NMR.

Key words: apiaceae; fructus carotae; arbutane; eudesmane; sesquiterpenoids
[1] Chinese Flora Editorial Board of Chinese Academy of Sciences Flora of China, Vol. 55, Science Press, Beijing, 1992, p. 223 (in Chinese).
(中国科学院中国植物志编辑委员, 中国植物志, 第55卷, 科学出版社, 北京, 1992, p. 223.)
[2] Zhang, H.; Gao, J. H.; Meng, L. J. Tianjin Med. Univ. 2004, 10, 492 (in Chinese).
(张卉, 高建华, 孟林, 天津医科大学学报, 2004, 10, 492.)
[3] Yang, R. L.; Yan, Z. H.; Lu, Y. J. Agric. Food Chem. 2008, 56, 3024.
[4] Pant, B.; Manandhar, S. Tumori 2010, 5, 461.
[5] Tavares, A. C.; Gonçalves, M. J.; Cavaleiro, C. J. Ethnopharmacol. 2008, 19, 129.
[6] Gebhardt, Y.; Witte, S.; Forkmann, G. Phytochemistry 2005, 66, 1273.
[7] Fu, H. W.; Zhang, L.; Yi, T.; Feng, Y. L.; Tian, J. K. Biochem. Syst. Ecol. 2010, 38, 309.
[8] Fu, Z. Z.; Han, H. T.; Liu, N.; Xu, X. B.; Zhu, W.; Gong, M. H.; Zhang, L.; Tian, J. K. Phytochem. Lett. 2015, 14, 35.
[9] Xiao, S. J.; Shi, D. B.; Yuan, Z. L.; Chen, Y. Z.; Zhang, M. S.; Ding, L. S.; Zhou, Y. Chin. J. Org. Chem. 2016, 36, 1686 (in Chinese).
(肖世基, 史大斌, 袁泽利, 陈永正, 张茂生, 有机化学, 2016, 36, 1686.)
[10] Liu, G. Y.; Wen, N.; Zhang, M. S.; Xu, Y. S.; Fu, S. B.; Xiao, S. J. Acta Pharm. Sinica 2017, 52, 1146 (in Chinese).
(刘贵园, 温楠, 张茂生, 徐应淑, 付少彬, 肖世基, 药学学报, 2017, 52, 1146.)
[11] Lee, I. K.; Lee, J. H.; Hwang, E. I.; Yun, B. S. Chem. Pharm. Bull. 2008, 56, 1483.
[12] Gao, X.; Deng, X. H. J. Chem. Res. 2009, 7, 457.
[13] Sun, Z. H.; Chen, B.; Zhang, S.; Hu, C. Q. J. Nat. Prod. 2004, 67, 1975.
[14] Brown, G. D.; Liang, G. Y.; Sy, L. K. Phytochemistry 2003, 64, 303.
[15] Xiao, S. J.; Chen, F.; Ding, L. S.; Zhou, Y. Chin. J. Nat. Med. 2015, 13, 65.
[16] Yang, M. C.; Lee, K. H.; Kim, K. H.; Choi, S. U.; Lee, K. R. Arch. Pharmacal. Res. 2007, 30, 1067.
[17] Kwak, Y. G.; Kim, D. K.; Ma, T. Z.; Park, S. A.; Park, H.; Jung, Y. H.; Yoo, D. J.; Eun, J. S. Arch. Pharmacal Res. 2006, 29, 834.
/
| 〈 |
|
〉 |