

2-甲基-1-取代苯基-2-丙胺类化合物的简便制备方法
收稿日期: 2018-01-21
修回日期: 2018-03-07
网络出版日期: 2018-04-04
基金资助
国家自然科学基金(No.21272029)资助项目.
An Efficient Method for the Synthesis of 2-Methyl-1-substituted-phenyl-2-propylamines
Received date: 2018-01-21
Revised date: 2018-03-07
Online published: 2018-04-04
Supported by
Project supported by the National Natural Science Foundation of China (No. 21272029).
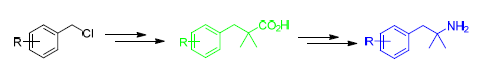
2-甲基-1-取代苯基-2-丙胺是合成β2肾上腺素受体激动剂类药物的重要中间体.以取代氯化苄为原料,通过与异丁腈发生烷基化反应,所得产物经过水解,Curtius重排和钯碳催化氢化反应,合成了一系列2-甲基-1-取代苯基-2-丙胺.此方法操作简便安全,原料经济易得,产率较高,可以适用于制备多种类型的2-甲基-1-取代苯基-2-丙胺类化合物.
关键词: 2-甲基-1-取代苯基-2-丙胺; β2肾上腺素受体激动剂; Curtius重排; 合成
朱兰平 , 赵帅 , 仓志鹏 , 周安迪 , 陈新 . 2-甲基-1-取代苯基-2-丙胺类化合物的简便制备方法[J]. 有机化学, 2018 , 38(7) : 1695 -1702 . DOI: 10.6023/cjoc201801029
2-Methyl-1-substituted-phenyl-2-propylamines are key intermediates for synthesizing the β2 adrenoceptor agonists. A series of 2-methyl-1-substituted-phenyl-2-propylamines have been synthesized starting from substituted benzyl chlorides. Isobutyronitrile was alkylated with benzyl chloride, and the resulting 2-methyl-1-aryl-2-butylcynide was hydrolyzed to give the corresponding acid. Curtius rearrangement of the acid and catalytic hydrolgenation of the resulting Cbz-protected amine afforded the title compound. The method is simple and safe to operate, the raw materials are readily available and the overall yields are satisfactory. This method is suitable for the synthesis of 2-methyl-2-(2-methylphenyl)-phenyl-2-propylamine and various kinds of derivatives.

[1] Pasternak, A; Goble, S. D.; Reynalda, K. Bioorg. Med. Chem. Lett. 2009, 19, 6237.
[2] Southers, J. A.; Bauman, J. N.; Kalgutkar, A. S. Med. Chem. Lett. 2010, 1, 219.
[3] Jesudason, C. D.; Trankle, W. G.; Shuker, A. J. Med. Chem. Lett. 2011, 2, 583.
[4] Czeskis, B. A.; Wheeler, W. J.; Clodfelter, D. K. J. Labelled Compd. Radiopharm. 2006, 49, 663.
[5] Tautermann, C. S.; Pautsch, A. Med. Chem. Lett. 2011, 2, 414.
[6] Hoenke, C.; Bouyssou, T.; Konetzki, I. Bioorg. Med. Chem. Lett. 2009, 19, 6640.
[7] Hoenke, C.; Bouyssou, T.; Konetzki, I. Bioorg. Med. Chem. Lett. 2010, 20, 1410.
[8] Goldfuss, B.; Denisenko, D.; Kulhanek, J. Chem.-Eur. J. 2004, 10, 4252.
[9] Ritter, J. J.; Minieri, P. P. J. Am. Chem. Soc. 1948, 70, 4045.
[10] Florvall, L.; Fagervall, I.; Ask, A.-L.; Ross, S. B. J. Med. Chem. 1986, 29, 2250.
[11] Wang, J. B.; Sun, X. Q.; Chen, X. Chin. J. Org. Chem. 2013, 33, 634 (in Chinese).
(王江波, 孙小强, 陈新, 有机化学, 2013, 33, 634.)
[12] Ling. P. X.; Fang, S. L.; Yin, X. S.; Chen, K.; Sun, B. Z.; Shi, B. F. Chem.-Eur. J. 2015, 21, 17503.
[13] Espino, C. G.; Fiori, K. W.; Bois, J. D. J. Am. Chem. Soc. 2004, 126, 15378.
[14] Ninomiya, K.; Shioiri, T.; Yamada, S. Tetrahedron 1974, 30, 2151.
[15] Jasys, V. J.; Lombardo, F.; Volkmann, R. A. J. Am. Chem. Soc. 2000, 122, 466.
[16] Fleming, F. F.; Ghosh, S.; Steward, O. W. J. Org. Chem. 2008, 73, 2803.
[17] Jiang, X.; Hartwig, J. F. Angew. Chem., Int. Ed. 2017, 56, 8887.
[18] Spectral data for the Curtius rearrangement intermediate 2-methyl-1-(2-methylphenyl)-2-propyl isocyanate:1H NMR (300 MHz, CDCl3) δ:7.18~7.17 (m, 4H), 2. 84 (s, 2H), 2.36 (s, 3H), 1.38 (s, 6H); 13C NMR (75 MHz, CDCl3) δ:137.34, 135.23, 131.59, 130.73, 127.11, 125.68, 122.84, 59.32, 45.31, 30.65, 20.38; HRMS (ESI, positive) calcd for C12H15NO[M+H]+ 190.1226, found 190.1306.
[19] Jung, M. E.; Amico, D. C. J. Am. Chem. Soc. 1995, 117, 7379.
/
| 〈 |
|
〉 |