

新型苯并噻吩稠合吡啶并[1,2-a]嘧啶衍生物的合成及其抑菌活性
收稿日期: 2018-01-08
修回日期: 2018-03-25
网络出版日期: 2018-04-12
基金资助
国家自然科学基金(No.31770688)、国家公益性行业专项(No.201404718)、哈尔滨市应用技术研究与开发资金(No.2017RAQXJ129)和黑龙江省卫生计生委科研基金(No.2017-579)资助项目.
Synthesis and Fungicidal Activity of Novel Benzothiophene-Fused Pyrido[1,2-a]pyrimidine Derivatives
Received date: 2018-01-08
Revised date: 2018-03-25
Online published: 2018-04-12
Supported by
Project supported by the National Natural Science Foundation of China (No. 31770688), the National Public Welfare Industry Special (No. 201404718), the Harbin Research and Development Fund of Applied Technology (No. 2017RAQXJ129) and the Scientific Research Fund of the Health Planning Committee of Heilongjiang (No. 2017-579).
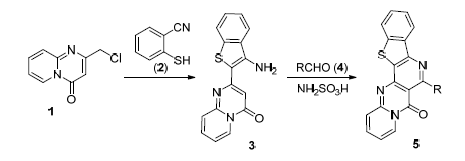
利用Thorpe-Ziegler反应,通过2-氯甲基-4H-吡啶并[1,2-a]嘧啶-4-酮与2-巯基苯甲腈的环化制得2-(3-氨基苯并噻吩-2-基)-4H-吡啶并[1,2-a]嘧啶酮,继而在氨基磺酸作用下,通过Pictet-Spengler反应,设计合成了新型苯并噻吩并[3',2':2,3]吡啶并[4,5-d]吡啶并[1,2-a]嘧啶衍生物.初步抑菌活性试验表明,当浓度为50 mg/L时,化合物5b对黄瓜灰霉病菌和小麦赤霉病菌的抑制率达96%以上,5f对油菜菌核病菌的抑制率为98%,5g和5i对烟草赤星病菌的抑制率达93%以上.
关键词: 吡啶并[1,2-a]嘧啶; 苯并噻吩; 2-巯基苯甲腈; Pictet-Spengler反应; 合成; 抑菌活性
徐姣 , 马玲 , 刘秀波 , 马伟 , 马岩 , 王道林 . 新型苯并噻吩稠合吡啶并[1,2-a]嘧啶衍生物的合成及其抑菌活性[J]. 有机化学, 2018 , 38(7) : 1680 -1686 . DOI: 10.6023/cjoc201801016
A series of novel benzothieno[3',2':2,3]pyrido[4,5-d]pyrido[1,2-a]pyrimidines are prepared via Pictet-Spengler reaction of 2-(3-aminobenzothiophene-2-yl)-4H-pyrido[1,2-a]pyrimidin-4-one using sulfamic acid as a catalyst, which in turn were obtained from the Thorpe-Ziegler isomerization of 2-(chloromethyl)-4H-pyrido[1,2-a]pyrimidin-4-one with 2-mercapto-benzonitrile. The structures of the products were characterized by FT-IR, 1H NMR, 13C NMR spectra and elemental analysis. The fungicidal activities of the prepared compounds were also preliminarily evaluated. For example, 5b exhibited more than 96% inhibition rate to Botrytis cinerea and Gibberella zeae at 50 mg/L, 5f exhibited 98% inhibition rate to Sclerotonia sclerotiorum at 50 mg/L, and 5g, 5i exhibited more than 93% inhibition rate to Alternaria alternata at 50 mg/L.

[1] (a) Buron, F.; Merour, J. Y.; Akssira, M.; Guillaumet, G.; Routier, S. Eur. J. Med. Chem., 2015, 95, 76.
(b) Dinakaran, V. S.; Bomma, B.; Srinivasan, K. K. Pharm. Chem. 2012, 4, 255.
(c) Wu, Q.; Song, J. B.; Jin, L. H.; Hu, D. Y. Chin. J. Org. Chem. 2009, 29, 365 (in Chinese).
(吴琴, 宋宝安, 金林红, 胡德禹, 有机化学, 2009, 29, 365.)
[2] Nakayama, K.; Kawato, H.; Watanabe, J.; Ohtsuka, M. Bioorg. Med. Chem. Lett. 2004, 14, 475.
[3] Kraker, A. J.; Hartl, B. G.; Amar, A. M.; Barvian, M. R. Biochem. Pharmacol. 2000, 60, 885.
[4] Jarvis, M. F.; Yu, H.; Wismer, C. T.; Zhu, C. Pain 2002, 96, 107.
[5] Helena, S.; Ladowska; A.; Sabiniarz, B. F. Farmaco 2003, 58, 25.
[6] (a) Wang, T.; Liu, X. Y.; Luo, J.; Xu, X. M.; Yu, D. H. Chin. J. Org. Chem. 2011, 31, 1773 (in Chinese).
(王涛, 刘雪英, 罗劲, 徐晓明, 于丹红, 有机化学, 2011, 31, 1773.)
(b) Ren, Q. Y.; Wang, T.; Liu, J. C.; He, H. W. Chin. J. Org. Chem. 2005, 25, 1530 (in Chinese).
(任青云, 王涛, 刘建超, 贺红武, 有机化学, 2005, 25, 1530.)
(c) Zhao, A. L.; Liu, Z.; Zhu, Y. M.; Wang, T.; Luo, J. Chin. J. Org. Chem. 2017, 37, 1877 (in Chinese).
(赵安林, 刘姝, 朱咏梅, 王涛, 罗劲, 有机化学, 2017, 37, 1877.)
(d) Song, P. P.; Li, N.; Cui, F.; Xin, J. C.; Zhang, X. S.; Cao, Q. P.; Wang, C. J.; Dai, W. J.; Meng, X. C.; Liu, M.; Chang, T. H.; Liu, J. Y.; Sun, Y. H.; Zhang, Q. R.; Liu, H. M. Chin. J. Org. Chem. 2017, 37, 2725 (in Chinese).
(宋攀攀, 栗娜, 崔飞, 辛景超, 张孝松, 曹钦坡, 王超杰, 戴文杰, 孟祥川, 刘梦, 常通航, 柳晴怡, 孙月红, 可钰, 张秋荣, 刘宏民, 有机化学, 2017, 37, 2725.)
[7] (a) Huang, J.; Luo, H.; Wang, L.; Guo, Y.; Zhang, W.; Chen, H.; Zhu, M.; Liu Y.; Yu, G. Org. Lett. 2012, 14, 3300.
(b) Ni, Y.; Nakajima, K.; Kanno K.; Takahashi, T. Org. Lett. 2009, 11, 3702.
[8] Romagnoli, R.; Baraldi, P. G.; Carrion, M. D.; Cara, C. L.; Preti, D.; Fruttarolo, F.; Pavani, M. G.; Tabrizi, M. A.; Tolomeo, M.; Grimaudo, S.; Cristina, A. D.; Balzarini, J.; Hadfield, J. A.; Brancale, A.; Hamel, E. J. Med. Chem. 2007, 50, 2273.
[9] Chonan, T.; Wakasugi, D.; Yamamoto, D.; Yashiro, M.; Oi, T.; Tanaka, H.; Ohoka-Sugita, A.; Io, F.; Koretsune H.; Hiratate, A. Bioorg. Med. Chem. 2011, 19, 1580.
[10] Berrade, L.; Aisa, B.; Ramirez, M. J.; Galiano, S.; Guccione, S.; Moltzau, L. R.; Levy, F. O.; Nicoletti, F.; Battaglia, G.; Molinaro, G.; Aldana, I.; Monge, A.; Perez-Silanes, S. J. Med. Chem. 2011, 54, 3086.
[11] Lee, K. C.; Moon, B. S.; Lee, J. H.; Chung, K. H.; Katzene-llenbogen, J. A.; Chi, D. Y. Bioorg. Med. Chem. 2003, 11, 3649.
[12] Vogel, V. G.; Costantino, J. P.; Wickerham, D. L.; Cronin, W. M.; Cecchini, R. S.; Atkins, J. N.; Bevers, T. B.; Fehrenbacher, L.; Pajon, E. R.; Wade, J. L.; Robidoux, A.; Margolese, R. G.; James, J.; Lippman, S. M.; Runowicz, C. D.; Ganz, P. A.; Reis, S. E.; McCaskill-Stevens, W.; Ford, L. G.; Jordan V. C.; Wolmark, N. JAMA, J. Am. Med. Assoc. 2006, 295, 2727.
[13] Lu, P.; Schrag, M. L.; Slaughter, D. E.; Raab, C. E.; Shou, M.; Rodrigues, A. D. Drug Metab. Dispos. 2003, 31, 1352.
[14] Croxtall, J. D.; Plosker, G. L. Drugs 2009, 69, 339.
[15] (a) Wang, D. L.; Wang, D.; Li, Q. M.; Qian, J. H. Heterocycles 2016, 92, 2244.
(b) Wang, D. L.; Wang, D.; Yan, L.; Pan, G. Y.; Yang, J. N. Heterocycles 2016, 92, 552.
(c) Wang, D. L.; Wang, D.; Yan, L.; Pan, G. Y.; Yang, J. N. Chin. Chem. Lett., 2016, 27, 953.
(d) Wang, D.; Wang, D. L.; Qian. J. H. Chin. J. Org. Chem. 2017, 37, 698 (in Chinese).
(王冬, 王道林, 钱建华, 有机化学, 2017, 37, 698.)
[16] (a) Litvinov, V. P.; Dotsenko, V. V.; Krivokolysko, S. G. Russ. Chem. Bull. 2005, 54, 864.
(b) Litvinov, V. P.; Dotsenko, V. V.; Krivokolysko, S. G. In Advances in Heterocyclic Chemistry, Ed.:Katritzky, A. R., Academic, New York, 2007, 93, 117.
[17] Böhme, H.; Weisel, K. H. Arch. Pharm. 1977, 310, 26.
[18] (a) Kamal, A.; Babu, K. S.; Hussaini, S. M. A.; Srikanth, P. S.; Balakrishna, M.; Alarifi, A. Tetrahedron Lett. 2015, 56, 4619.
(b) Zhou J. F.; Gong, G. X.; An, L. T.; Sun, X. J.; Zhu. F. X. Chin. J. Org. Chem. 2009, 29, 1988 (in Chinese).
(周建峰, 贡桂霞, 安礼涛, 孙小军, 朱凤霞, 有机化学, 2009, 29, 1988.)
/
| 〈 |
|
〉 |