

基于二硫代氨基甲酸酯活性亚结构的先导优化及其抗增殖活性
收稿日期: 2018-01-02
修回日期: 2018-03-15
网络出版日期: 2018-04-13
基金资助
国家自然科学基金(No.201502063)、湖北省自然科学基金(No.2016CFB562)资助项目.
Lead Optimization and Antiproliferative Activity Using a New Dithiocarbamates Substructure
Received date: 2018-01-02
Revised date: 2018-03-15
Online published: 2018-04-13
Supported by
Project supported by the National Natural Science Foundation of China (No. 201502063) and the Natural Science Foundation of Hubei Province (No. 2016CFB562).
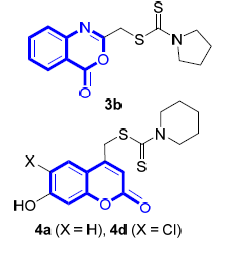
设计了一系列含喹唑啉酮、苯并噁嗪酮和香豆素单元的二硫代氨基甲酸酯(DTC)衍生物.基于收率高、反应时间短、条件温和易于后处理的三组分一锅法反应,可高效构建目标化合物库.采用四甲基偶氮唑盐(MTT法)测试了目标化合物对人肝癌细胞HCCLM-7、人宫颈癌细胞Hela、人乳腺癌细胞MDA-MB-435S、人结肠癌细胞SW-480、人喉癌细胞Hep-2和人乳腺癌细胞MCF-7等6种肿瘤细胞株的体外抗增殖活性.结果表明,3个化合物表现出高效和广谱的抗增殖活性(IC50:3.5~13.5μmol·L-1).部分化合物的活性较阳性对照药5-氟尿嘧啶(5-FU)提高了10倍以上(IC50:8.1~128.7μmol·L-1).以上结果表明,含稠杂环单元的二硫代氨基甲酸酯(DTC)衍生物是一类有价值的抗肿瘤活性先导结构.
孙海燕 , 孙宏顺 , 刘明珍 , 黄伟 , 杨光富 . 基于二硫代氨基甲酸酯活性亚结构的先导优化及其抗增殖活性[J]. 有机化学, 2018 , 38(8) : 2067 -2075 . DOI: 10.6023/cjoc201801001
In this work, a series of dithiocarbamate derivatives bearing diverse quinazolinones, benzoxazinones, and coumarin moieties were designed and synthesized via a one-pot three-component reaction. These compounds produced good yields and functioned quickly under mild conditions, and the desired products were readily isolated. Their in vitro antitumor activities were evaluated by the methyl thiazolyl tetrazolium (MTT) method against hepatoma carcinoma cells HCCLM-7, cervical carcinoma cells Hela, mammary adenocarcinoma cells MDA-MB-435S, colon carcinoma cells SW-480, laryngocarcinoma cells Hep-2, and mammary adenocarcinoma cells MCF-7. 3 compounds were identified as the most promising candidates, due to their high potency and broad-spectrum antiproliferative activity (IC50:3.5~13.5 μmol·L-1). The activities of some lead compounds were more than 10-fold more potent than that of positive control 5-fluorouracil (5-FU) (IC50:8.1~128.7 μmol·L-1). These results indicated that the dithiocarbamate (DTC) derivatives bearing fused heterocyclic moieties could be used as lead for further developing new antitumor active compounds.

Key words: dithiocarbamate; quinazolinone; benzoxazinone; coumarin; antiproliferative activity
[1] Li, R.-D.; Wang, Y.-Q.; Ge, Z.-M.; Li, R.-T. Chin. J. Org. Chem. 2015, 35, 1805(in Chinese). (李日东, 王元强, 葛泽梅, 李润涛有机化学, 2015, 35, 1805.)
[2] Bala, V.; Gupta, G.; Sharma, V. L. Mini-Rev. Med. Chem. 2014, 14, 1021.
[3] Room, R.; Babor, T.; Rehm, J. Lancet 2005, 365, 519.
[4] Sauna, Z. E.; Shukla, S.; Ambudkar, S. V. Mol. Biosyst. 2005, 1, 127.
[5] Schenk, H.; Klein, M.; Erdbrügger, W.; Dröge, W.; Schul-ze-Osthoff, K. Proc. Natl. Acad. Sci. U. S. A. 1994, 91, 1672.
[6] Gaudernak, E.; Seipelt, J.; Triendl, A.; Grassauer, A.; Kuechler, E. J. Virol. 2002, 76, 6004.
[7] Kraljevic, T. G.; Harej, A.; Sedic, M.; Pavelic, S. K.; Stepanic, V.; Drenjancevic, D.; Talapko, J.; Raic-Malic, S. Eur. J. Med. Chem. 2016, 124, 794.
[8] Mo, S.; Ding, Y.; Zhang, G.; Zhang, Z.; Shao, X.-B.; Li, Q.-Y.; Yang, X.-J.; Chen, F. Chin. J. Org. Chem. 2017, 37, 1000(in Chinese). (莫松, 丁勇, 张刚, 张震, 杓学蓓, 李清寒, 杨学军, 陈峰, 有机化学, 2017, 37, 1000.)
[9] (a) Fu, D. J.; Zhang, S. Y.; Liu, Y. C.; Zhang, L.; Liu, J. J.; Song, J.; Zhao, R. H.; Li, F.; Sun H. H.; Liu, H. M.; Zhang, Y. B. Bioorg. Med. Chem. Lett. 2016, 26, 3918.
(b) Ding, P. P.; Gao, M.; Mao, B. B.; Cao, S. L.; Liu, C. H.; Yang, C. R.; Li, Z. F.; Liao, J.; Zhao, H. C.; Li, Z. Eur. J. Med. Chem. 2016, 108, 364.
(c) Fu, D. J.; Zhang, L.; Song, J.; Mao, R. W.; Zhao, R. H.; Liu, Y. C.; Hou, Y. H.; Li, J. H.; Yang J. J.; Jin, C. Y.; Li, P.; Zi, X. L.; Liu, H. M.; Zhang, S. Y.; Zhang, Y. B. Eur. J. Med. Chem. 2017, 127, 87.
(d) Jian, F.-F.; Xu, L.-Z.; Wen, L.-R.; Zhu, C.-Y. Chin. J. Org. Chem. 2005, 25, 423(in Chinese). (建方方, 许良忠, 文丽荣, 朱崇毅, 有机化学, 2005, 25, 423.)
[10] Carta, F.; Aggarwal, M.; Maresca, A.; Scozzafava, A.; McKenna, R.; Masini, E.; Supuran, C. T. J. Med. Chem. 2012, 55, 1721.
[11] Qian, Y.; Ma, G. Y.; Yang, Y.; Chen, K.; Zheng, Q. Z.; Mao, W. J.; Shi, L.; Zhao, J.; Zhu, H. L. Bioorg. Med. Chem. 2010, 18, 4310.
[12] Zheng, Y. C.; Duan, Y. C.; Ma, J. L.; Xu, R. M.; Zi, X.; Lv, W. L.; Wang, M. M.; Ye, X. W.; Zhu, S.; Mobley, D.; Zhu, Y. Y.; Wang, J. W.; Li, J. F.; Wang, Z. R.; Zhao, W.; Liu, H. M. J. Med. Chem. 2013, 56, 8543.
[13] Li, R. D.; Zhang, X.; Li, Q. Y.; Ge, Z. M.; Li, R. Bioorg. Med. Chem. Lett. 2011, 21, 3637.
[14] Zheng, Y. C.; Wang, L. Z.; Zhao, L. J.; Zhao, L. J.; Zhan, Q. N.; Ma, J. L.; Zhang, B.; Wang, M. M.; Wang, Z. R.; Li, J. F.; Liu, Y.; Chen, Z. S.; Shen, D. D.; Liu, X. Q.; Ren, M.; Lv, W. L.; Zhao, W.; Duan, Y. C.; Liu, H. M. Cell. Physiol. Biochem. 2016, 38, 185.
[15] Kamal, A.; Sathish. M., N ayak, V. L.; Srinivasulu, V.; Kavitha, B.; Tangella, Y.; Thummuri, D.; Bagul, C.; Shankaraiah, N., N agesh, N. Bioorg. Med. Chem. 2015, 23, 5511.
[16] Gaspari, P.; Banerjee, T.; Malachowski, W. P.; Muller, A. J.; Prendergast, G. C.; DuHadaway, J.; Bennett, S.; Donovan, A. M. J. Med. Chem. 2006, 49, 684.
[17] Buac, D.; Schmitt, S.; Ventro, G.; Kona, F. R.; Dou, Q. P. Mini-Rev. in Med. Chem. 2012, 12, 1193.
[18] Khan, I.; Zaib, S.; Batool, S.; Abbas, N.; Ashraf Z.; Iqbal, J.; Saeed, A. Bioorg. Med. Chem. 2016, 24, 2361.
[19] Macías, F. A.; Marín, D.; Oliveros-Bastidas, A.; Molinillo, J. M. Nat. Prod. Rep. 2009, 26, 478.
[20] Medina, F. G.; Marrero, J. G.; Macías-Alonso, M.; González, M. C.; Córdova-Guerrero, I.; Teissier García, A. G.; Osegueda-Robles, S. Nat. Prod. Rep. 2015, 32, 1472.
[21] Sandhu, S.; Bansal, Y.; Silakari, O.; Bansal, G. Bioorg. Med. Chem. 2014, 22, 3806.
[22] Huang, W.; Ding, Y.; Miao, Y.; Yang, G. F. Eur. J. Med. Chem. 2009, 44, 3687.
[23] Huang, W.; Chen, Q.; Yang, W. C.; Yang, G. F. Eur. J. Med. Chem. 2013, 66, 161.
[24] Huang, W.; Liu, M. Z.; Li, Y.; Tan, Y.; Yang, G. F. Bioorg. Med. Chem. 2007, 15, 5191.
[25] Liu, G.; Song, B. A.; Sang, W. J.; Yang, S.; Jin, L. H.; Ding, X. Chin. J. Org. Chem. 2004, 24, 1296(in Chinese). (刘刚, 宋宝安, 桑维钧, 杨松, 金林红, 丁雄, 有机化学, 2004, 24, 1296.)
[26] Clemence, F.; Le Martret, O.; Collard, J. J. Heterocycl. Chem. 1984, 21, 1345.
[27] Leonetti, F.; Favia, A.; Rao, A.; Aliano, R.; Paluszcak, A.; Hartmann, R. W.; Carotti, A. J. Med. Chem. 2004, 27, 6792.
[28] Li, R. T.; Cai, M. S. Synth. Commun. 1998, 28, 295.
[29] (a) Full crystallographic details has been deposited at the Cambridge Crystallographic Data Center and allocated the deposition number CCDC 715987(2h).
(b) Sheldrick, G. M. SHELXTL (Version 5. 0), University of Gottingen, Germany, 2001.
(c) Bruker SMART V5.628, SAINT V6.45. & SADABS Bruker AXS Inc., Madison, Wisconsin, USA, 2001.
[30] Mosmann, T. J. Immunol. Methods 1983, 65, 55.
/
| 〈 |
|
〉 |