

砷亚胺配位的高自旋亚铁配合物的合成、表征和乃春转移反应
收稿日期: 2018-03-12
修回日期: 2018-04-04
网络出版日期: 2018-04-13
基金资助
科技部国家重点研发计划(No.2016YFA0202900)、国家自然科学基金(Nos.51534004,21725104,21690062,21432001,U1362110)和中国科学院战略先导科技专项(No.XDB20000000)资助项目.
Synthesis, Structure, and Nitrene-Transfer Reactivity of High-Spin Iron(Ⅱ) Complex Featuring Iminoarsorane Ligation
Received date: 2018-03-12
Revised date: 2018-04-04
Online published: 2018-04-13
Supported by
Project supported by the National Key Research and Development Program of the Ministry of Science and Technology (No. 2016YFA0202900), the National Natural Science Foundation of China (Nos. 51534004, 21725104, 21690062, 21432001, U1362110) and the CAS Strategic Pilot Science and Technology Special (No. XDB20000000).
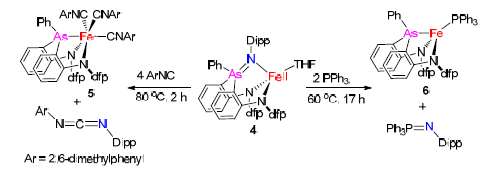
通过原位形成的胺基取代苯基锂与PhAsCl2反应合成二胺基-胂配体,并进一步由二胺基-胂配体与[Fe(N(TMS)2)2]2反应合成了(二胺基-胂)亚铁配合物[(κ-N,N,As-dfpN2As) Fe(THF)2](3).通过配合物3与有机叠氮化物DippN3反应实现了首例砷亚胺金属配合物[(κ-N,N,N-dfpN2AsNDipp) Fe(THF)](4)的合成.配合物4可与2,6-二甲基苯基异腈以及三苯基膦发生乃春转移反应,生成配合物[(κ-N,N,As-dfpN2As) Fe(CNC6H3Me2-2,6)3](5)和[(κ-N,N,As-dfpN2As) Fe(PPh3)](6)以及相应的有机物产物DippNCNC6H3Me2-2,6和Ph3PCNC6H3Me2-2,6.这些配合物通过了各种谱学手段表征,其中配合物3~5的结构还通过单晶X射线衍射得到确认.
赵明晶 , 毛国梁 , 刘洋 , 肖洁 , 邓亮 . 砷亚胺配位的高自旋亚铁配合物的合成、表征和乃春转移反应[J]. 有机化学, 2018 , 38(7) : 1656 -1662 . DOI: 10.6023/cjoc201803015
Treatment of (2,6-F2C6H3)(2-BrC6H4)NH with 2 equiv. of nBuLi, followed by the interaction with 0.5 equiv. of PhAsCl2 and quenching with aqueous solution, afforded the new bis(amido)-arsorane ligand ((o-(N-(2,6-2C6H3)NH)C6H4)2AsPh (denoted as H2(dfpN2As)). The interaction of H2(dfpN2As)) with 0.5 equiv. of[Fe(N(TMS)2)2]2 resulted in amine elimination and gave the bis(amido)-arsorene-iron(Ⅱ) complex[(κ-N,N,P-dfpN2As)Fe(THF)2] (3). Further treatment of 3 with the organic azide DippN3 rendered the formation of the first iminoarsorane-transition-metal complex[(κ-N,N,N-dfpN2AsNDipp)Fe(THF)] (4) as an air- and moisture-sensitive green solid. Reactivity study indicated that 4 can undergo nitrene-transfer reactions with excess amounts of 2,6-dimethylphenylisocyanide and PPh3 to produce the nitrene-transfer products DippNCNC6H3Me2-2,6 and Ph3PCNC6H3Me2-2,6, respectively and the corresponding bis(amido)-arsorane-iron(Ⅱ) complexes[(κ-N,N,As-dfpN2As)Fe(CNC6H3Me2-2,6)3] (5) and[(κ-N,N,As-dfpN2As)Fe(PPh3)] (6). These complexes were fully characterized by various spectroscopic methods. Solution magnetic susceptibility measurements and 57Fe Mössbauer spectroscopy (δ=0.88 mm/s,|ΔEQ|=1.50 mm/s; δ=0.90 mm/s,|ΔEQ|=2.53 mm/s; and δ=0.65 mm/s,|ΔEQ|=2.23 mm/s for 3, 4, and 6, respectively) indicated that 3, 4 and 6 are high-spin iron(Ⅱ) complexes, whereas the well-resolved diamagnetic NMR spectra of 5 in combination with its 57Fe Mössbauer spectrum data (δ=0.05 mm/s,|ΔEQ|=0.40 mm/s) revealed its low-spin iron(Ⅱ) nature. The molecular structures of 3~5 have been characterized by single-crystal X-ray diffraction studies. The dianionic bis(amido)-arsorane ligands in 3 and 5 chelate to the metal centers in a fac-fashion with the Fe-As distances being 0.2562(3) and 0.2293(1) nm, respectively. The dianionic bis(amido)-iminoarsorane ligand in 4 binds to the iron(Ⅱ) center in a fac-fashion. The Fe-As distance (0.285 nm) observed in 4 is longer than the sum of the covalent radii of Fe and As, indicating the absence of strong Fe-As interaction in the iminoarsorane-iron(Ⅱ) complex. Its As-N distance of 0.1752(3) nm is found comparable to those of the As-N bonds in the reported iminoarsoranes and bis(arsoranylidene) ammonium compounds.

Key words: arsorane; iminoarsorane; iron; nitrene; group-transfer
[1] Patai, S. The Chemistry of Organic Arsenic, Antimony and Bismuth Compounds, Wiley, Chichester, U. K., 1994.
[2] García-Álvarez, J.; García-Garrido, S. E.; Cadierno, V. J. Organomet. Chem. 2014, 751, 792.
[3] Roesky, H. W.; Witt, M.; Clegg, W.; Isenberg, W.; Noltemeyer, M.; Sheldric, G. M. Angew. Chem., Int. Ed. Engl. 1980, 19, 943.
[4] Matano, Y.; Nomura, H.; Suzuki, H.; Shiro, M.; Nakano, H. J. Am. Chem. Soc. 2001, 123, 10954.
[5] Matano, Y.; Nomura, H.; Suzuki, H. Inorg. Chem. 2002, 41, 1940.
[6] Nitta, M.; Mitsumoto, Y.; Yamamoto, H. J. Chem. Soc., Perkin Trans. 1 2001, 1901.
[7] Xiao, J.; Deng, L. Dalton Trans. 2013, 42, 5607.
[8] Liu, J.; Hu, L.; Wang, L.; Chen, H.; Deng, L. J. Am. Chem. Soc. 2017, 139, 3876.
[9] Howell, J. A. S.; Palin, M. G.; McArdle, P.; Cunningham, D.; Goldschmidt, Z.; Gottlieb, H. E.; Hezroni-Langerman, D. Inorg. Chem. 1993, 32, 3493.
[10] Song, L. C.; Hu, Q. M.; Zhou, Z. Y.; Hu, G. Z.; Xiang, Z. Y. Chin. J. Inorg. Chem. 1991, 7, 399 (in Chinese).
(宋礼成, 胡青眉, 周忠远, 胡国志, 向在筠, 无机化学学报, 1991, 7, 399.)
[11] El-khateeb, M.; Al-Noaimi, M.; Al-Akhras, A.; Görls, H.; Weigan, W. J. Coord. Chem. 2012, 65, 2510.
[12] Enemark, J. H.; Feltham, R. D.; Huie, B. T.; Johnson, P. L.; Swedo, K. B. J. Am. Chem. Soc. 1977, 99, 3285.
[13] Roesk, H. W.; Bertel, N.; Edelmann, F.; Noltemeyer, M.; Sheldrick G. M. Z. Naturforsch. 1988, 43b, 72.
[14] Koketsu, J.; Ninomiya, Y.; Suzuki, Y.; Koga, N. Inorg. Chem. 1997, 36, 694.
[15] Sudhakar, P. V.; Lammertsma, K. J. Am. Chem. Soc. 1991, 113, 1899.
[16] Sazama, G. T.; Betley, T. A. Organometallics 2011, 30, 4315.
[17] Hosokawa, S.; Ito, J.; Nishiyama, H. Organometallics 2012, 31, 8283.
[18] Peddarao, T.; Baishya, A.; Barman, M. K.; Kumara, A.; Nembenna, S. New J. Chem. 2016, 40, 7627.
[19] Chan, K. T. K.; Spencer, L. P.; Masuda, J. D.; McCahill, J. S. J.; Wei, P.; Stephan, D. W. Organometallics 2004, 23, 381.
[20] Betz, R.; Reichvilse, M. M.; ESchumi, E.; Miller, C.; Klüfers, P. Z. Anorg. Allg. Chem. 2009, 635, 1204.
[21] Olmstead, M. M.; Power, P. P.; Shoner, S. C. Inorg. Chem. 1991, 30, 2547.
[22] Guisado-Barrios, G.; Bouffard, J.; Donnadieu, B.; Bertrand, G. Angew. Chem., Int. Ed. 2010, 49, 4759.
[23] Evans, D. F. J. Chem. Soc. 1959, 2003.
[24] Sur, S. K. J. Magn. Reson. 1989, 82, 169.
/
| 〈 |
|
〉 |