

Hydramicromelins A,B和C母核的对映选择性合成
收稿日期: 2018-02-09
修回日期: 2018-03-28
网络出版日期: 2018-04-27
基金资助
国家自然科学基金(Nos.21372205,21302175)和河南省科技厅基础研究(No.132300410028)资助项目.
Enantioselective Synthesis of Core Structures of Hydramicromelins A, B and C
Received date: 2018-02-09
Revised date: 2018-03-28
Online published: 2018-04-27
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21372205, 21302175) and the Basic Research Project of Science and Technology Department of Henan Province (No. 132300410028).
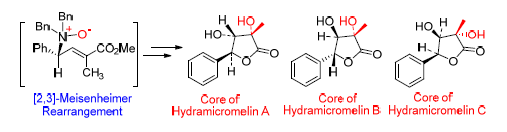
Hydramicromelins A~C是具有独特化学结构和生物活性的香豆素类化合物.以本实验室发展的[2,3]-Meisenheimer重排为关键反应,以L-苯甘氨醇为起始原料,经过Wittig反应,[2,3]-Meisenheimer重排,环氧化,双羟化等7~8步反应,高收率、高对映选择性地制备出Hydramicromelins A,B和C的母核或其对映体.
孙默然 , 代磊 , 杨华 , 刘宏民 , 于德泉 . Hydramicromelins A,B和C母核的对映选择性合成[J]. 有机化学, 2018 , 38(9) : 2443 -2449 . DOI: 10.6023/cjoc201802017
Hydramicromelins A~C are coumarin compounds with unique chemical structure and biological activity. With[2,3]-Meisenheimer rearrangemen as a key reaction which has been developed in our laboratory, the core structures of Hydramicromelin A, B and C were synthesized from L-phenylglycine. The route included Wittig reaction,[2,3]-Meisenheimer rearrangement, epoxidation and dihydroxylation reaction, and it was high-yield and high-enantioselectivity.

[1] Lambert, J. A.; Price, J. R.; Redcliffe, A. H. Aust. J. Chem. 1967, 20, 973.
[2] Cassady, J. M.; Ojima, N.; Chang, C. J.; Mclaughlin, J. L. J. Nat. Prod. 1979, 42, 274.
[3] He, H. P.; Zou, Y.; Shen, Y. M.; Hao, X. J. Chin. Chem. Lett. 2001, 12, 603.
[4] Huo, X.; Ren, X.; Xu, Y.; Li, X.; She, X.; Pan, X. Tetrahedron:Asymmetry 2008, 19, 343.
[5] Yang, H.; Sun, M.; Zhao, S.; Zhu, M.; Xie, Y.; Niu, C.; Li, C. J. Org. Chem. 2013, 78, 339.
[6] (a) Xie, Y.; Sun, M.; Zhou, H.; Cao, Q.; Gao, K.; Niu, C.; Yang, H. J. Org. Chem. 2013, 78, 10251.
(b) Sun, M.; Xie, Y.; Gu, J.; Yang, H. Can. J. Chem. 2013, 91, 738.
(c) Gao, K.; Sun, M.; Zhu, M.; Zhou, H.; Cao, Q.; Yang, H. Chin. J. Org. Chem. 2013, 33, 1939.
(d) Sun, M.; Gao, K.; Zheng, J.; Lai, Y.; Yang, H. Res. Chem. Intermed. 2015, 41, 1181.
(d) Sun, M.; Li, Y.; Shi, X.; Niu, C.; Yang, H. ARKIVOC 2016, 4, 172.
[7] (a) Mosa, F.; Thirsk, C.; Vaultier, M.; Maw, G.; Whiting, A. Org. Synth. 2008, 85, 219.
(b) Denis, J. N.; Correa, A.; Greene, A. E. J. Org. Chem. 1991, 56, 6939.
[8] (a) Fujii, T.; Itaya, T.; Matsubara, S. Chem. Pharm. Bull. 1989, 37, 1758.
(b) Pihko, P. M.; Koskinen, A. M. J. Org. Chem. 1998, 63, 92.
[9] Hoffmann, R. W. Chem. Rev. 1989, 89, 1841.
[10] (a) Jiang, B.; Chen, Z.; Tang, X. Org. Lett. 2002, 4, 3451.
(b) Infante, R.; Nieto, J.; Andrés, C. Chem.-Eur. J. 2012, 18, 4375.
(c) Blay, G.; Fernández, I.; Marco-Aleixandre, A.; Pedro, J. R. Org. Lett. 2006, 8, 1287.
[11] (a) Zhang, X.; Sun, X.; Tan, J.; Fan, H.; Rao, W. Chin. J. Org. Chem. 2015, 35, 2049(in Chinese). (中文作者, 有机化学, 2015, 35, 2049.)
(b) Zhang, X.; Teo, J.; Ma, D.; Leung, C.; Chan, P. Tetrahedron Lett. 2014, 55, 6703.
[12] Wu, H.; Yang, B.; Zhu, L.; Lu, R.; Li, G.; Lu, H. Org. Lett. 2016, 18, 5804.
/
| 〈 |
|
〉 |