

红树林真菌Penicillium camemberti OUCMDZ-1492产生的细菌群体感应抑制活性的α-吡喃酮类化合物
收稿日期: 2018-03-13
修回日期: 2018-04-24
网络出版日期: 2018-05-03
基金资助
国家自然科学基金-广东联合基金(No.U1501221)、国家自然科学基金(Nos.81561148012,81373298)资助项目.
α-Pyronoids with Quorum Sensing Inhibitory Activity from the Mangrove Fungus Penicillium camemberti OUCMDZ-1492
Received date: 2018-03-13
Revised date: 2018-04-24
Online published: 2018-05-03
Supported by
Project supported by the National Natural Science Foundation of China-Guangdong Fund Joint Project (No. U1501221) and the National Natural Science Foundation of China (Nos. 81561148012, 81373298).
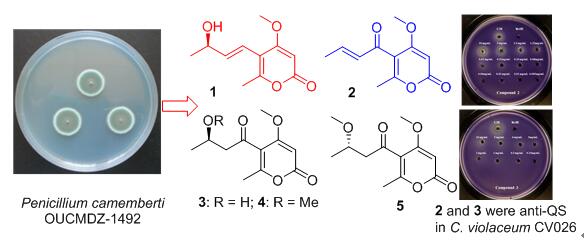
正红树根泥来源的沙门柏干酪青霉(Penicillium camemberti) OUCMDZ-1492在寡营养条件下能够产生一类不同于富营养条件下的代谢产物,具有细菌群体感应抑制活性.采用活性跟踪的分离方法并对其中的外消旋体进行手性拆分,得到了5个α-吡喃酮类化合物;通过波谱分析、电子圆二色谱(ECD)的测量与化学模拟计算,其结构分别被鉴定为:(R,E)-6-甲基-5-(3-羟基-1-丁烯基)-4-甲氧基-2-吡喃酮(1,命名为pyrenocine P)、pyrenocine A(2)、(R)-pyrenocine B(3)、(R)-(—)-pyrenocine E(4)和(S)-(+)-pyrenocine E(5),其中化合物1为新化合物、化合物3~5的绝对构型为首次确定.化合物2和3对紫色杆菌(Chromobacterium violaceum) CV026显示出较强的群体感应抑制活性,最小抑制浓度MIC分别为0.16和1.0 mg·mL-1[阳性药(Z)-4-溴-5-溴亚甲基-2-呋喃酮(C-30)的MIC为0.08 mg·mL-1].
樊亚琴 , 朱国良 , 王乂 , 朱晓翠 , 宫倩红 , 贾茜 , 付鹏 , 朱伟明 . 红树林真菌Penicillium camemberti OUCMDZ-1492产生的细菌群体感应抑制活性的α-吡喃酮类化合物[J]. 有机化学, 2018 , 38(10) : 2798 -2804 . DOI: 10.6023/cjoc201803017
The mangrove fungus Penicillium camemberti OUCMDZ-1492 produced a new class of compounds under the oligotrophic condition, which were different from those under the eutrophic condition. Bioassay-guided isolation and chiral resolution for the racemic mixture resulted in the harvest of five α-pyronoids. Their structures were ideinfied as (R,E)-5-(3-hydroxybut-1-en-1-yl)-4-methoxy-6-methyl-2H-pyran-2-one (1), pyrenocine A (2), (R)-pyrenocine B (3), (R)-(-)-pyrenocine E (4) and (S)-(+)-pyrenocine E (5). Their structures, including absolute configurations, were elucidated on the basis of spectral analysis, electronic circular dichroism (ECD) spectra, quantum chemical calculation, and chiral separation. New compound 1 was named as pyrenocine P, and the absolute configurations of compounds 3~5 were determined for the first time. Compounds 2 and 3 showed potent quorum sensing inhibitory activity in Chromobacterium violaceum CV026 with the minimal inhibitory concentrations of 0.16 and 1.0 mg·mL-1, respectively. The minimum inhibitory concentration (MIC) value of (Z)-4-bromo-5-(bromomethylene)-furan-2 (5H)-one (C-30), a positive control, was 0.08 mg·mL-1.

[1] Jiang, Y.-X.; Zheng, T.-L.; Tian, Y. Acta Microbiol. Sin. 2006, 46, 848(in Chinese). (蒋云霞, 郑天凌, 田蕴, 微生物学报, 2006, 46, 848.)
[2] Liu, F.; Hong, K. Hainan Med. J. 2006, 17, 171(in Chinese). (刘峰, 洪葵, 海南医学, 2006, 17, 171.)
[3] Zhao, C.; Zhu, T.; Zhu, W. Chin. J. Org. Chem. 2013, 33, 1195(in Chinese). (赵成英, 朱统汉, 朱伟明, 有机化学, 2013, 33, 1195.)
[4] Bugni, T. S.; Ireland, C. M. Nat. Prod. Rep. 2004, 21, 143.
[5] (a) Elias, B. C.; Said, S.; de Albuquerque, S.; Pupo, M. T. Microbiol. Res. 2006, 161, 273.
(b) Schauer, R.; Bienhold, C.; Alban, R.; Harder, J. ISME J. 2010, 4, 159.
[6] Lin, Y.; Wang, L.; Wang, Y.; Wang, W.; Hao, J.; Zhu, W. Chin. J. Org. Chem. 2015, 35, 1955(in Chinese). (林亚伟, 王立平, 王乂, 王伟, 郝杰杰, 朱伟明, 有机化学, 2015, 35, 1955.)
[7] Chen, L.; Zhu, T.; Zhu, G.; Liu, Y.; Wang, C.; Piyachaturawat, P.; Chairoungdua, A.; Zhu, W. Chin. J. Org. Chem. 2017, 37, 2752(in Chinese). (陈玲玲, 朱统汉, 朱国良, 刘云龙, 王聪, Pawinee Piyachaturawat, Arthit Chairoungdua, 朱伟明, 有机化学, 2017, 37, 2752.)
[8] Fan, Y.; Wang, Y.; Liu, P.; Fu, P.; Zhu, T.; Wang, W.; Zhu, W. J. Nat. Prod. 2013, 76, 1328.
[9] Amagata, T.; Minoura, K.; Numata, A. J. Antibiot. 1998, 51, 432.
[10] Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Montgomery, J. A.; Jr., Vreven, T.; Kudin, K. N.; Burant, J. C.; Millam, J. M.; Iyengar, S. S.; Tomasi, J.; Barone, V.; Mennucci, B.; Cossi, M.; Scalmani, G.; Rega, N.; Petersson, G. A.; Nakatsuji, H.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Klene, M.; Li, X.; Knox, J. E.; Hratchian, H. P.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Ayala, P. Y.; Morokuma, K.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Zakrzewski, V. G.; Dapprich, S.; Daniels, A. D.; Strain, M. C.; Farkas, O.; Malick, D. K.; Rabuck, A. D.; Raghavachari, K.; Foresman, J. B.; Ortiz, J. V.; Cui, Q.; Baboul, A. G.; Clifford, S.; Cioslowski, J.; Stefanov, B. B.; Liu, G.; Liashenko, A.; Piskorz, P.; Komaromi, I.; Martin, R. L.; Fox, D. J.; Keith, T.; Al-Laham, M. A.; Peng, C. Y.; Nanayakkara, A.; Challacombe, M.; Gill, P. M. W.; Johnson, B.; Chen, W.; Wong, M. W.; Gonzalez, C.; Pople, J. A. Gaussian 03, Revision E.01, Gaussian, Inc., Wallingford CT, 2004.
[11] Cammi, R.; Tomasi, J. J. Comput. Chem. 1995, 16, 1449.
[12] Gross, E. K. U.; Dobson, J. F.; Petersilka, M. In Density Functional Theory Ⅱ, Vol. 181, Ed.:Nalewajski, R. F., Springer-Verlag Berlin Heidelberg, 1996, p. 81.
[13] Wang, L.; Zhou, S.; Yin, S.; Liu, H.; Yu, W.; Gong, Q. Biotechnol. Lett. 2011, 33, 1381.
[14] Zou, H.-X.; Yang, Q.-M. Occup. Health 2006, 22, 1928(in Chinese). (邹红霞, 杨庆民, 职业与健康, 2006, 22, 1928.)
[15] Slesak, G.; Douangdala, P.; Inthalad, S.; Silisouk, J.; Voungsouath, M.; Sengduangphachanh, A.; Moore, C. E.; Mayxay, M.; Matsuoka, H.; Newtoncorresponding, P. N. Ann. Clin. Microbiol. Antimicrob. 2009, 8, 24.
[16] Sirinavin, S.; Techasaensiri, C.; Benjaponpitak, S.; Pornkul, R.; Vorachit, M. Pediatr. Infect. Dis. J. 2005, 24, 559.
[17] Ichihara, A.; Murakami, K.; Sakamura, S. Tetrahedron 1987, 43, 5245.
[18] Foongladda, S.; Roengsanthia, D.; Arjrattanakool, W.; Chuchottaworn, C.; Chaiprasert, A.; Franzblau, S. G. Int. J. Tuberc. Lung D 2002, 6, 1118.
[19] Tominaga, H.; Ishiyama, M.; Ohseto, F.; Sasamoto, K.; Hamamoto, T.; Suzuki, K.; Watanabe, M. Anal. Commun. 1999, 36, 47.
/
| 〈 |
|
〉 |