

基于全氟烷基磺酰亚胺催化剂合成、固载及催化应用研究进展
收稿日期: 2018-01-08
修回日期: 2018-03-30
网络出版日期: 2018-05-03
基金资助
江苏省自然科学基金(No.BK20140969)、江苏高校优势学科建设工程(PAPD)和江苏省生物质能源与材料重点实验室开放基金(No.JSBEM201603)资助项目.
Research Progress of the Synthesis, Immobilization Bis(perfluoro-alkylsulfonyl)imide-Based Complexes and Application in Heterogeneous Catalysis
Received date: 2018-01-08
Revised date: 2018-03-30
Online published: 2018-05-03
Supported by
Project supported by the Natural Science Foundation of Jiangsu Province (No. BK20140969), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and the Open Fund of Jiangsu Key Laboratory of Biomass Energy and Materials (No. JSBEM201603).
洪梅 , 闵洁 , 王石发 . 基于全氟烷基磺酰亚胺催化剂合成、固载及催化应用研究进展[J]. 有机化学, 2018 , 38(8) : 1907 -1916 . DOI: 10.6023/cjoc201801013
Traditional liquid acid catalysts have environmental problems during their application. Perfluoroalkylsulfonylimide complexes show a wide application in catalysis due to their special anionic structures and super acid properties. The structures and properties of bis(perfluoroalkylsulfonyl)imide complexes are introduced. The synthesis and immobilization methods of bis(perfluoroalkylsulfonyl)imide complexes are summarized. The use of immobilized bis(perfluoroalkylsulfonyl)imide complexes in catalytic reactions is also reviewed. The development trends of perfluoroalkylsulfonylimide complexes in the catalytic application are prospected.
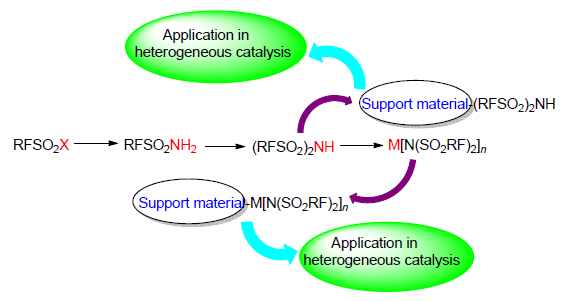
Key words: bis(perfluoroalkylsulfonyl)imide; synthesis; immobilization; catalysis
[1] (a) Yamamoto, H. Lewis Acids in Organic Synthesis, Vols. 1& 2, Wiley-VCH, Weinheim, 2000, p. 994.
(b) Santelli, M.; Pons, J. M. Selectivity in Lewis Acid Promoted Reactions, CRC, Baton Rouge, 1995, p. 352.
(c) Akiyama, T. Chem. Rev. 2007, 107, 5744.
(d) Yamamoto, H.; Ishihara, K. Acid Catalysis in Modern Organic Synthesis, Vols. 1& 2, Wiley-VCH, Weinheim, 2008, p. 1662.
[2] (a) Xiao, J.; Zhang, Z.; Nie, J. Prog. Chem. 2005, 17, 137(in Chinese). (肖杰展, 张正波, 聂进, 化学进展, 2005, 17, 137.)
(b) Zhan, X.; Tang, Y.; He, Y.; Zhang, Q.; Chen, F. J. Chem. Eng. Chin. Univ. 2016, 253(in Chinese). (詹晓力, 唐永强, 何逸波, 张庆华, 陈丰秋, 高校化学工程学报, 2016, 253.)
[3] (a) Bochmann, M. Angew. Chem., Int. Ed. Engl. 1992, 31, 1181.
(b) Strauss, S. H. Chem. Rev. 1993, 93, 927.
(c) Seppelt K. Angew. Chem., Int. Ed. Engl. 1993, 32, 1025.
[4] (a) Leito, I.; Raamat, E.; Kütt, A.; Saame, J.; Kipper, K.; Koppel, I. A.; Zhang, M.; Mishima, M.; Yagupolskii, L. M.; Garlyauskayte, R. Y.; Filtov, A. A. J. Phys. Chem. A 2009, 113, 8421.
(b) KoppeI, I. A.; Taft, R. W.; Anvia, F.; Zhu, S.; Hu, L.; Sung, K.; DesMarteau, D. D.; Yagupolskii, L. M.; Yagupolskii, Y. L.; Ignat'ev, N. V.; Kondratenko, N. V.; Volkonskii, A. Y.; Vlasov, V. M.; Notario, R.; Maria P. J. Am. Chem. Soc. 1994, 116, 3047.
[5] (a) Hong, M.; Yao, M.; Pan, H. RSC Adv. 2015, 5, 91558.
(b) Hong, M.; Xiao, G. J. Fluorine Chem. 2012, 140, 121.
(c) Hong, M.; Xiao, G. J. Fluorine Chem. 2013, 146, 11.
[6] Ruff, J. K. Inorg. Chem. 1965, 4, 1446.
[7] Meussdorffer, J. N.; Niederprum, H. Chem.-Ztg. 1972, 96, 582.
[8] (a) Foropoulos, J. J. R.; DesMarteau, D. D. Inorg. Chem. 1984, 23, 3720.
(b) DesMarteau, D. D.; Witz, M. J. Fluorine Chem. 1991, 52, 7.
[9] (a) Foropoulos, J.; DesMarteau, D. D. J. Am. Chem. Soc. 1982, 104, 4260.
(b) Hu, L. Q.; DesMarteau, D. D. Inorg. Chem. 1993, 32, 5007.
(c) Geiculescu, O. E.; Yang, J.; Blau, H.; Bailey-Walsh, R.; Creager, S. E.; Pennington, W. T.; DesMarteau, D. D. Solid State Ionics 2002, 148, 173.
(d) Singh, S.; DesMarteau, D. D. Inorg. Chem. 1990, 29, 2982.
(e) Xue, L. X.; Padgett, C. W.; DesMarteau, D. D. Solid State Sci. 2002, 4, 1535.
[10] (a) Kobayashi, H.; Nie, J.; Sonoda, T. Chem. Lett. 1995, 24, 307.
(b) Mikami, K.; Kotera, O.; Motoyama, Y.; Sakaguchi, H. Synlett. 1995, 975.
(c) Hao, X. H.; Yoshida, A.; Nishikido, J. J Fluorine Chem. 2006, 127, 193.
[11] (a) Kannan, K.; Choi, J. W.; Iseki, N.; Senthilkumar, K.; Kim, D. H.; Giesy, J. P. Chemosphere 2002, 49, 225.
(b) Kannan, K.; Corsolini, S.; Falandysz, J.; Oehme, G.; Focardi, S.; Giesy, J. P. Sci. Technol. 2002, 36, 3210.
(c) Kannan, K.; Newsted, J.; Halbrook, R. S.; Giesy, J. P. En-viron. Sci. Technol. 2002, 36, 2566.
[12] (a) Olsen, G. W.; Church, T. R.; Miller, J. P.; Burris, J. M.; Hansen, K. J.; Lundberg, J. K.; Armitage, J. B.; Herron, R. M.; Medhdizadehkashi, Z.; Nobiletti, J. B.; O'Neill, E. M.; Mandel, J. H.; Zobel, L. R. Environ. Health Perspect. 2003, 111, 1892.
(b) Olsen, G. W.; Logan, P. W.; Hansen, K. J.; Simpson, C. A.; Burris, J. M.; Burlew, M. M.; Vorarath, P. P.; Venkateswarlu, P.; Schumpert, J. C.; Mandel, J. H. AIHA J. 2003, 64, 651.
(c) Olsen, G. W.; Church, T. R.; Larson, E. B.; van Belle, G.; Lundberg, J. K.; Hansen, K. J.; Burris, J. M.; Mandel, J. H.; Zobel, L. R. Chemosphere 2004, 54, 1599.
(d) Lehmler, H. J. Chemosphere 2005, 58, 1471.
[13] (a) Roesky, H. W.; Holtschneider, G.; Giere, H. H. Z. Naturforsch 1970, 25b, 252.
(b) Meuβdoerffer, J. N.; Niederprüm, H. Chem.-Ztg. 1972, 96, 582.
(c) Bussas, R.; Kresze, G. Liebigs Ann. Chem. 1982, 545.
(d) Podol'skii, A. V.; Kachalkova, M. I.; Ilatovskii, R. E.; Kodess, M. I.; Kolenko, I. P. Russ. J. Org. Chem. 1990, 1242.
[14] Foropoulos, J. J. R.; DesMarteau, D. D. Inorg. Chem. 1984, 23, 3720.
[15] (a) Beyer, H.; Thieme, E. J. Prakt. Chem. 1966, 31, 293.
(b) Volkov, N. D.; Nazaretyan, V. P.; Yagupol'skii, L. M. Syn-thesis 1979, 972.
(c) Kamigata, N.; Kawakita, O.; Izuoka, A.; Kobayashi, M. J. Org. Chem. 1985, 50, 398.
(d) Zhu, S.-Z. Tetrahedron Lett. 1992, 33, 6503.
(e) Xu, Y.; Zhu, S. Tetrahedron 1999, 55, 13725.
[16] Zhu, S.-Z.; Xu, Y.; Wang, Y.-L.; Peng, W.-M. Chin. J. Chem. 2001, 19, 1259.
[17] Lehmler, H. J.; Rao, R.; Nauduri, D.; Vargo, J. D.; Parkin, S. J. Fluorine Chem. 2007, 128, 595.
[18] Benfodda, Z.; Delon, L.; Guillen, F.; Blancou, H. J. Fluorine Chem. 2007, 128, 1353.
[19] Hong, M.; Cai, C.; Yi, W. J. Fluorine Chem. 2010, 131, 111.
[20] (a) Zhou, Z.; Han, H.; Fu, S.; Chen, H. CN 102617414, 2012[Chem. Abstr. 2012, 157, 345510].
(b) Han, H.; Zhou, Y.; Liu, K.; Nie, J.; Huang, X.; Armand, M.; Zhou, Z. Chem. Lett. 2010, 39, 472.
[21] Michot, C.; Armand, M.; Gauthier, M.; Choquette, Y. US 6319428, 2001.
[22] Howells, R. D.; Lamanna, W. M.; Fanta, A. D.; Waddell, J. US 5874616, 1999[Chem. Abstr. 1999, 130, 184069].
[23] Sogabe, K.; Hasegawa, Y.; Wada, Y.; Kitamura, T.; Yanagid, S. Chem. Lett. 2000, 944.
[24] (a) Iwahori, T.; Mitsuishi, I.; Shiraga, S.; Nakajima, N.; Momose, H.; Ozaki, Y.; Taniguchi, S.; Awata, H.; Ono, T.; Takeuchi, K. Electrochim. Acta 2000, 45, 1509.
(b) Wang, X.; Yasukawa, E.; Kasuya, S. J. Electrochem. Soc. 2000, 147, 2421.
(c) Broussely, M.; Biensan, P.; Simon, B. Electrochim. Acta 1999, 45, 3.
[25] (a) Peyronneau, M.; Arrondo, C.; Vendier, L.; Roques, N.; Le Roux, C. J. Mol. Catal. A 2004, 211, 89.
(b) Repichet, S.; Zwick, A.; Vendier, L.; Le Roux, C.; Dubac, J. Tetrahedron Lett. 2002, 43, 993.
(c) Baudry, D. B.; Dormond, A.; Duris, F.; Bernard, J. M.; Desmurs, J. R. J. Fluorine Chem. 2003, 121, 233.
(d) Earle, M. J.; Hakala, U.; McAuley, B. J.; Nieuwenhuyzen, M.; Ramani, A.; Seddon, K. R. Chem. Commun. 2004, 1368.
[26] Baudrya, D. B.; Dormonda, A.; Durisa, F.; Bernardb, J. M.; Desmursc, J. R. J. Fluorine Chem. 2003, 121, 233.
[27] Xue, L.; Padgett, C. W.; DesMarteau, D. D.; Pennington, W. T. Solid State Sci. 2002, 4, 1535.
[28] (a) Vij, A.; Zheng, Y. Y.; Kirchmeier, R. L.; Shreeve, J. M. Inorg. Chem. 1994, 33, 3281.
(b) Rogers, E. I.; Silvester, D. S.; Ward Jones, S. E.; Aldous, L.; Hardacre, C.; Russell, A. J.; Davies, S. G.; Compton, R. G. J. Phys. Chem. C 2007, 111, 13957.
(c) Serizawa, N.; Katayama, Y.; Miura, T. Electrochim. Acta 2010, 56, 346.
(d) Agel, F.; Pitsch, F.; Krull, F. F.; Schulz, P.; Wessling, M.; Melin, T.; Wasserscheid, P. Phys. Chem. Chem. Phys. 2011, 13, 725.
(e) Williams, D. B.; Stoll, M. E.; Scott, B. L.; Costa, D. A.; Oldham, W. J. Chem. Commun. 2005, 1438.
(f) Arvai, R.; Toulgoat, F.; Langlois, B. R.; Sanchez, J.-Y.; Médebielle, M. Tetrahedron 2009, 65, 5361.
(g) Stricker, M.; Oelkers, B.; Rosenau, C. P.; Sundermeyer, J. Chem.-Eur. J. 2013, 19, 1042.
[29] Arvai, R.; Toulgoat, F.; Langlois, B. R.; Sanchez, J.; Médebielle, M. Tetrahedron 2009, 65, 5361.
[30] (a) Strauss, S. H.; Polyakov, O. G.; Hammel, J. W.; Ivanova, S. M.; Ivanov, S. V.; Havighurst, M. D. US 6114266, 2000[Chem. Abstr. 2000, 133, 228539].
(b) Polyakov, O. G.; Ivanova, S. M.; Gaudinski, C. M.; Miller, S. M.; Anderson, O. P.; Strauss, S. H. Organometallics 1999, 18, 3769.
[31] Haas, A.; Klare, C.; Betz, P.; Bruckmann, J.; Kruger, C.; Tsay, Y. H.; Aubke, F. Inorg. Chem. 1996, 35, 1918.
[32] Picot, A.; Repichet, S.; Le Roux, C.; Dubac, J.; Roques, N. J. Fluorine Chem. 2002, 116, 129.
[33] Antoniotti, S.; Duñach, E. Chem. Commun. 2008, 993.
[34] Shibuya, M.; Fujita, S.; Abe, M.; Yamamoto, Y. ACS Catal. 2017, 7, 2848.
[35] (a) Takasu, A.; Makino, T.; Yamada, S. Macromolecules 2010, 43, 144.
(b) Qiu, T.; Xu, X.; Qian, X. J. Chem. Technol. Biotechnol. 2009, 84, 1051.
[36] Tan, E.; Ung, S.; Corbet, M. Eur. J. Org. Chem. 2016, 1836.
[37] Liu, F.; De Oliveira Vigier, K.; Pera-Titus, M.; Pouilloux, Y.; Clacens, J.; Decampo, F.; Jérôme F. Green Chem. 2013, 15, 901.
[38] Ponra, S.; Vitale, M. R.; Michelet, V.; Ratovelomanana-Vidal, V. J. Org. Chem. 2015, 80, 3250.
[39] Zhao, Y.; Hu, Y.; Wang, C.; Li, X.; Wan, B. J. Org. Chem. 2017, 82, 3935.
[40] Horváth, T. H.; Rabai, J. Science 1994, 266, 72.
[41] Horváth, I, T. Acc. Chem. Res. 1998, 31, 641.
[42] (a) Nishikido, J.; Nakajima, H.; Saeki, T.; Ishii, A.; Mikami, K. Synlett 1998, 1347.
(b) Hao, X. H.; Yoshida, A.; Nishikido, J. Tetrahedron Lett. 2005, 46, 2697.
(c) Hao, X. H.; Yoshida, A.; Nishikido, J. Green Chem. 2004, 6, 566.
(d) Hao, X. H.; Hoshi, N. Chem Lett. 2006, 35, 1102.
[43] Hao, X. H.; Yamazaki, O.; Yoshida, A.; Nishikido, J. Tetrahedron Lett. 2003, 44, 4977.
[44] Wang, L.; Nie, J.; Li, X.; Zhang, Z.; Yin, F. Chin. J. Org. Chem. 2004, 24, 778(in Chinese). (王丽琼, 聂进, 李小永, 张正波, 尹飞, 有机化学, 2004, 24, 778.)
[45] Schager, F.; Bonrath, W. Appl. Catal A:Gen. 2000, 202, 117.
[46] Nishikido, J.; Nasayuki, N.; Yoshida, A.; Nakajima, H.; Matsuoto, Y.; Mikami, K. Synlett 2002, 1613.
[47] Xiao, J. Z.; Zhang, Z. B.; Nie, J. J. Mol. Catal. A:Chem. 2005, 236, 119.
[48] (a) Tzschucke, C. C.; Andrushko, V.; Bannwarth, W. Eur. J. Org. Chem. 2005, 5248.
(b) Hensle, E. M.; Bannwarth, W. Helv. Chim. Acta 2012, 95, 2072.
(c) Hong, M.; Xiao, G. J. Fluorine Chem. 2012, 144, 7.
[49] Yamazaki, O.; Hao, X. H.; Yoshida, A.; Nishikido, J. Tetrahedron Lett. 2003, 44, 8791.
[50] (a) Hoshino, M.; Degenkolb, P.; Curran, D. P. J. Org. Chem. 1997, 62, 8341.
(b) Berendsen, G. E., Galan, L. D. J. Liquid Chromatogr. 1978, 1, 403.
(c) Billiet, H. A. H.; Schoenmakers, P. J.; De Galan, L. J. Chromatogr. 1981, 218, 443.
(c) Berendsen, G. E.; Pikaart, K. A.; De Galan, L.; Olieman, C. Anal. Chem. 1980, 52, 1990.
(d) Sadek, P. C.; Carr, P. W. J. Chromatogr. 1984, 288, 25.
(e) Kainz, S.; Luo, Z. Y.; Curran, D. P.; Leitner, W. Synthesis 1998, 1425.
[51] Yamazaki, O.; Hao, X. H.; Yoshida, A.; Nishikido, J. Tetrahedron Lett. 2003, 44, 8791.
[52] Hong, M.; Xiao, G. J. Fluorine Chem. 2013, 146, 11.
[53] Yuan, Y. B.; Nie, J.; Zhang, Z. B.; Wang, S. J. Appl. Catal. A:Gen. 2005, 295, 170.
[54] Yang, Q.; Ma, Z.; Ma, J.; Nie, J. Microporous Mesoporous Mater. 2013, 172, 51.
[55] Chen, M.; You, L.; Zhang, H.; Ma, Z. Catal. Lett. 2016, 146, 2165.
[56] Ma, Z.; Han, H.; Zhou, Z.; Nie, J. J. Mol. Catal. A:Chem. 2009, 311, 46.
[57] Jiang, H. Curr. Org. Chem. 2005, 9, 289.
[58] Nishikido, J.; Kamishima, M.; Matsuzawa, H.; Mikami, K. Tetrahedron 2002, 58, 8345.
[59] (a) Rogers, R. D.; Seddon, K. Science 2003, 302, 792.
(b) Lee, S. G. Chem. Commun. 2006, 1049.
(c) Earle, M. J.; Esperança, J. M. S. S.; Gilea, M. A.; Lopes, J. N. C.; Rebelo, L. P. N.; Magee, J. W.; Seddon, K. R.; Widegre, J. A. Nature 2006, 439, 831.
[60] Wang, S. J.; Jiang, S. J.; Nie, J. Adv. Synth. Catal. 2009, 351, 1939.
/
| 〈 |
|
〉 |