

金属镍催化炔基铝与炔溴高效合成对称1,3-二炔化合物
收稿日期: 2018-02-14
修回日期: 2018-04-04
网络出版日期: 2018-05-17
基金资助
西南民族大学研究生创新基金(No.CX2016SZ063)和四川省科厅科技支撑(No.2015NZ0033)资助项目.
Synthesis of Symmetrical 1, 3-Diynes via Cross-Coupling Reaction of Alkynyl Bromide with Alkynyl Aluminum Catalyzed by Nickel
Received date: 2018-02-14
Revised date: 2018-04-04
Online published: 2018-05-17
Supported by
Project supported by the Postgraduate Degree Construction Project of Southwest University for Nationalities (No. CX2016SZ063) and the Sichuan Provincial Department of Science and Technology Support Program (No. 2015NZ0033).
张刚 , 杓学蓓 , 李清寒 , 杨学军 . 金属镍催化炔基铝与炔溴高效合成对称1,3-二炔化合物[J]. 有机化学, 2018 , 38(6) : 1538 -1543 . DOI: 10.6023/cjoc201802019
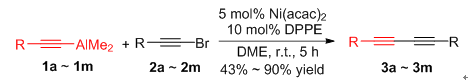
1,3-Diyne compounds are important inter mediates in organic synthesis, which are widely used in pharmaceutical chemistry, organic synthesis and materials science. A highly efficient for the synthesis of 1,3-diyne via cross-coupling of alkynyl bromides with alkynyl aluminium reagents catalyzed by nickel has been developed. The coupling reaction of alkynyl bromides with alkynyl aluminium reagents mediated by Ni(acac)2 (5 mol%)/1,2-bis(diphenylphosphino)ethane nickel(Ⅱ) chloride (DPPE) (10 mol%)in 1,2-dimethoxyethane afforded the corresponding coupling products 1,3-diyne in good to excellent yields(up to 90%) at room temperature for 5 h. The coupling reaction of alkynyl aluminum with different substituents and alkynyl bromine with various substituents can afforded the coupling products in good yields. Importantly, the α-ethynylnaphthalene and 2-ethynylthiophene were also suitable for the reaction. This process is simple and easily performed, which provides an efficient method for the synthesis of 1,3-diynes derivatives.
Key words: 1,3-diyne; nickel; alkynyl bromide; alkynyl aluminium; cross-coupling
[1] Shun, S. A. L.; Tykwinski, R. R. Angew.Chem., Int.Ed. 2006, 45, 1034.
[2] Zhou, Y. Z.; Ma, H. Y.; Chen, H.; Qiao, L.; Yao, Y.; Cao, J. Q.; Pei, Y. H. Chem.Pharm.Bull. 2006, 54, 1455.
[3] Eisler, S.; Slepkov, A. D.; Elliott, E.; Luu, T.; McDonald, R.; Hegmann, F. A.; Tykwinski, R. R. J.Am.Chem. Soc. 2005, 127, 2666.
[4] Sienmsen, P.; Livingston, R. C.; Diederich, F. Angew. Chem., Int.Ed. 2000, 39, 2632.
[5] Diederich, F.; Stang, P. J.; Tykwinski, R. R. Acetylene Chemistry:Chemistry, Biology and Material Science, Wiley-VCH Verlag Gmbh & Co. KGAa, Weinheim, Germany, 2005.
[6] Haro, T.; Nevado, C. J.Am.Chem. Soc. 2010, 132, 1512.
[7] Lerch, M. L.; Harper, M. K.; Faulkner, D. J. J. Nat.Prod. 2003, 66, 667.
[8] Stütz, A. Angew.Chem., Int. Ed. 1987, 26, 320.
[9] Morandi, S.; Pellati, F.; Benvenuti, S.; Prati, F. Tetrahedron 2008, 64, 6324.
[10] Xiao, R.; Yao, R.; Cai, M. Eur.J.Org.Chem. 2012, 4178.
[11] Glaser, C. Ber.Dtsch.Chem.Ges. 1869, 2, 422.
[12] (a) Bai, D. H.; Li, C. J.; Li, J.; Jia, X. S. Chin.J. Org.Chem. 2012, 32, 994(in Chinese). (白东虎, 李春举, 李健, 贾学顺, 有机化学, 2012, 32, 994.)
(b) Shi, W.; Lei, A. W. Tetrahedron Lett. 2014, 55, 2763.
[13] (a) Feng, X. J.; Zhao, Z. R.; Yang, F.; Jin, T. N.; Ma, Y. J.; Bao, M. J.Organomet.Chem. 2011, 696, 1479.
(b) Zhu, Y.; Shi, Y. Org.Biomol.Chem. 2013, 11, 7451.
(c) Fan, X. H.; Li, N.; Shen, T.; Cui, X. M.; Lv, H.; Zhu, H. B.; Guan, Y. H. Tetrahedron 2014, 70, 256.
(d) Chen, Z. G.; Jiang, H. F.; Wang, A. Z.; Yang, S. R. J. Org. Chem. 2010, 75, 6700.
[14] Kim, Y.; Park, A.; Park, K.; Lee, S. W. Tetrahedron Lett. 2011, 52, 1766.
[15] (a) Li, J. X.; Liang, H. R.; Wang, Z. Y.; Fu, J. H. Monatsh.Chem. 2011, 142, 507. (b) Perrone, S.; Bona, F.; Troisi, L. Tetrahedron 2011, 67, 7386.
(c) Yang, Z. P.; Wang, B. N.; Xu, X. L.; Wang, H.; Li, X. N. Chin.J. Org.Chem. 2015, 35, 207(in Chinese). (杨振平, 王兵南, 许孝良, 王红, 李小年, 有机化学, 2015, 35, 207.)
(d) Liu, Y. Y.; Wang, C. P.; Wang, X. B.; Wan, J. P. Tetrahedron Lett. 2013, 54, 3953.
[16] (a) Zhu, Y. G.; Xiong, T.; Han, W. Y.; Shi, Y. A. Org.Lett. 2014, 16, 6144.
(b) Singh, F. V.; Amaral, M. F. Z. J.; Stefani, H. A. Tetrahedron Lett. 2009, 50, 2636.
[17] Liu, D. X.; Li, F. L.; Li, H. X.; Gao, J.; Lang, J. P. Tetrahedron 2014, 70, 2416.
[18] Dermenci, A.; Whittaker R. E.; Dong, G. Org.Lett. 2013, 15, 2242.
[19] (a) Shrestha, B.; Thapa, S.; Gurung, S. K.; Pike, R. A. S.; Giri, R. J. Org. Chem. 2016, 81, 787.
(b) Crepin, D. F.; Harrity, J. P. A. Org. Lett. 2013, 15, 4222.
(c) Li, Q. H.; Shao, X. B.; Zhang, G.; Ding, Y.; Yang, X. J.; Chen, F. Chin.J.Org.Chem. 2018, 38, 802(in Chinese). (李清寒, 杓学蓓, 张刚, 丁勇, 杨学军, 陈峰, 有机化学, 2018, 38, 802.)
(d) Feuvrie, C.; Blanchet, J.; Bonin, M.; Micouin, L. Org Lett. 2004, 6, 2333.
(e) Shen, Z. L.; Peng, Z. H.; Yang, C. M.; Helberg, J.; Mayer, P.; Marek, I.; Knochel, P. Org.Lett. 2014, 16, 956.
(f) Blümke, T. D.; Groll, K.; Karaghiosoff, K.; Knochel, P. Org.Lett. 2011, 13, 6440.
[20] (a) Chinchilla, R.; Nijera, C. Chem. Rev. 2007, 107, 874.
(b) Negishi, E-I.; Anastasia, L. Chem. Rev. 2003, 103, 1979.
[21] (a) Zhang, Z.; Mo, S.; Zhang, G.; Shao, X. B.; Li, Q. H.; Zhong, Y. Synlett 2017, 28, 611.
(b) Zhang, Z.; Shao, X. B.; Zhang, G.; Li, Q. H.; Li, X. Y. Synthesis 2017, 49, 3643.
(c) Mo, S.; Shao, X. B.; Zhang, G.; Li, Q. H. RSC Adv. 2017, 7, 27243.
(d) Ding, Y.; Li, Q. H.; Zhao, Z. G.; Yang, X. J.; Chen, F. Chin.J. Org.Chem. 2017, 37, 3282(in Chinese) (丁勇, 李清寒, 赵志刚, 杨学军, 陈峰, 有机化学, 2017, 37, 3282.)
/
| 〈 |
|
〉 |