

钌亚丙二烯基配合物与肼的反应性质:丙烯腈配合物的生成
收稿日期: 2018-04-13
修回日期: 2018-05-14
网络出版日期: 2018-05-17
基金资助
国家重点基础研究发展计划(973计划,No.2012CB821600)和长江学者和创新团队发展计划(No.IRT_17R65).
Reactivity of Ruthenium Allenylidene Complexes with Hydrazines:Formation of Acrylonitrile Complexes
Received date: 2018-04-13
Revised date: 2018-05-14
Online published: 2018-05-17
Supported by
Project supported by the National Basic Research Program of China (973 Program, No. 2012CB821600) and the Program for Changjiang Scholars and Innovative Research Team in University (No. IRT_17R65).
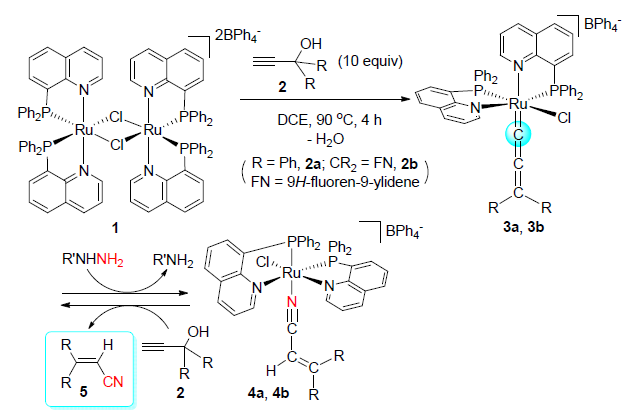
以双齿P,N-配体8-(二苯基膦基)喹啉(DPPQ)为支撑配体的钌亚丙二烯基配合物[RuCl(=C=C=CR2)(DPPQ)2]-[BPh4](3a:R=苯基;3b:CR2=FN=亚芴基)可由双核钌配合物[Ru(μ-Cl)(DPPQ)2]2[BPh4]2(1)分别与过量的1,1-二苯基炔丙醇(2a)或9-乙炔-9-芴醇(2b)反应得到.配合物3易与肼在室温下反应生成丙烯腈的钌配合物[RuCl(N≡C-CH=CR2)(DPPQ)2][BPh4](4a:R=苯基;4b:CR2=FN=亚芴基),该反应涉及肼对亚丙二烯基配体α-碳原子的分子间亲核进攻,是首例肼对金属亚丙二烯基加成生成丙烯腈的反应.配合物4与过量的丙炔醇2反应可释放出3,3-二苯基丙烯腈(5a)或3-芴基丙烯腈(5b),并再生亚丙二烯基配合物3.此外,初步考察了配合物1对端基炔丙醇与肼反应生成丙烯腈的催化活性,结果表明该催化反应的确可以进行,但是得到的丙烯腈产物的产率不高.尽管结果不是很理想,但是这些研究表明可望发展端基炔丙醇与肼经由过渡金属亚丙二烯基中间体转化为丙烯腈的新催化反应.
蔡涛 , 杨玉 , 张丽 , 温庭斌 . 钌亚丙二烯基配合物与肼的反应性质:丙烯腈配合物的生成[J]. 有机化学, 2018 , 38(8) : 2017 -2027 . DOI: 10.6023/cjoc201804023
The cationic ruthenium allenylidene complexes[RuCl(=C=C=CR2)(DPPQ)2] [BPh4] (3a:R=Ph; 3b:CR2=FN=9H-fluoren-9-ylidene) supported by the heterobidentate P,N-donor ligand 8-(diphenylphosphanyl)quinoline (DPPQ) have been synthesized from the reactions of the dimeric complex[Ru(μ-Cl)(DPPQ)2]2[BPh4]2 (1) with excess 1,1-diphenylprop-2-yn-1-ol (2a) or 9-ethynyl-9H-fluoren-9-ol (2b), respectively. Addition of hydrazines to the ruthenium-allenylidenes 3 led to the facile formation of ruthenium-bound acrylonitrile complexes[RuCl(N≡C-CH=CR2)(DPPQ)2] [BPh4] (4a:R=Ph; 4b:CR2=FN) at room temperature. This reaction involves the intermolecular nucleophilic attack of hydrazines at the Cα atom of the allenylidene ligand, which represents the first examples of addition of hydrazines to metal-allenylidenes affording acrylonitrile derivatives. Reaction of acrylonitrile complex 4 with an excess of propargyl alcohols 2a or 2b (4 equiv.) could release the organic acrylonitriles 3,3-diphenylacrylonitrile (5a) or 2-(9H-fluoren-9-ylidene)-acetonitrile (5b) along with regeneration of allenylidene complex 3. In addition, the catalytic activity of 1 for the transformation of terminal propargyl alcohols and hydrazines into acrylonitriles has been investigated preliminarily. The results showed that the catalytic reaction did proceed to give the desired acrylonitrile products, albeit the yield not good. Nevertheless, our results of the catalytic reactions demonstrated that it is very promised to develop new catalytic reactions for the transformation of terminal propargylic alcohols and hydrazines into acrylonitriles via allenylidene intermediates.

Key words: ruthenium; allenylidene; alkyne; hydrazine; nitrile
[1] (a) Bruce, M. I. Chem. Rev. 1998, 98, 2797.
(b) Herndon, J. W. Coord. Chem. Rev. 2018, 356, 1 and references therein.
(c) Cadierno, V.; Gimeno, J. Chem. Rev. 2009, 109, 3512.
(d) Che, C. M.; Ho, C. M.; Huang, J. S. Coord. Chem. Rev. 2007, 251, 2145.
(e) Varela, J. A.; González-Rodríguez, C.; Saá, C. Top. Organ-omet. Chem. 2014, 48, 237.
(f) Lozano-Vila, A. M.; Monsaert, S.; Bajek, A.; Verpoort, F. Chem. Rev. 2010, 110, 4865.
(g) Nishibayashi, Y. Synthesis 2012, 44, 489.
(h) Rigaut, S.; Touchard, D.; Dixneuf, P. H.; Coord. Chem. Rev. 2004, 248, 1585.
(i) Bruneau, C.; Dixneuf, P. H. Angew. Chem., Int. Ed. 2006, 45, 2176.
[2] Selected examples:(a) Hyder, I.; Jimenez-Tenorio, M.; Puerta, M. C.; Valerga, P. Organometallics 2011, 30, 726.
(b) Smith, E. J.; Johnson, D. G.; Thatcher, R. J.; Whitwood, A. C.; Lynam, J. M. Organometallics 2013, 32, 7407.
(c) Bruce, M. I.; Burgun, A.; Fox, M. A.; Jevric, M.; Low, P. J.; Nicholson, B. K.; Parker, C. R.; Skelton, B. W.; White, A. H.; Zaitseva, N. N. Organometallics 2013, 32, 3286.
(d) Alós, J.; Bolaño, T.; Esteruelas, M. A.; Oliván, M.; Oñate, E.; Valencia, M. Inorg. Chem. 2014, 53, 1195.
(e) Garcia de la Arada, I.; Díez, J.; Gamasa, M. P.; Lastra, E. Organometallics 2015, 34, 1345.
(f) Schauer, P. A.; Skelton, B. W.; Koutsantonis, G. A. Organometallics 2015, 34, 4975.
(g) Spoerler, S.; Strinitz, F.; Rodehutskors, P.; Mueller, L.; Waterloo, A. R.; Duerr, M.; Huebner, E.; Ivanovic-Burmazovic, I.; Tykwinski, R. R.; Burzlaff, N. New J. Chem. 2016, 40, 2167.
(h) Jimenez-Tenorio, M.; Puerta, M. C.; Valerga, P. Organometallics 2016, 35, 388.
(i) Huang, J. B.; Zhou, X. X.; Zhao, Q. Y.; Li, S. H.; Xia, H. P. Chin. J. Chem. 2017, 35, 420.
[3] Coletti, C.; Marrone, A.; Re, N. Acc. Chem. Res. 2012, 45, 139.
[4] (a) Cadierno, V.; Gamasa, M. P.; Gimeno, J.; González-Cueva, M.; Lastra, E.; Borge, J.; García-Granda, S.; Pérez-Carreńo, E. Organometallics 1996, 15, 2137.
(b) Auger, N.; Touchard, D.; Rigaut, S.; Halet, J.-F.; Saillard, J.-Y. Organometallics 2003, 22, 1638.
(c) Wong, C.-Y.; Lai, L.-M.; Lam, C.-Y.; Zhu, N. Organometallics 2008, 27, 5806.
[5] Selected examples:(a) Pino-Chamorro, J. A.; Bustelo, E.; Puerta, M. C.; Valerga, P. Organometallics 2009, 28, 1546.
(b) Bustelo, E.; Jimenez-Tenorio, M.; Puerta, M. C.; Valerga, P. Organometallics 2007, 26, 4300.
(c) Rigaut, S.; Touchard, D.; Dixneuf, P. H. Organometallics 2003, 22, 3980.
(d) Bustelo, E.; Jiménez-Tenorio, M.; Mereiter, K.; Puerta, M. C.; Valerga, P. Organometallics 2002, 21, 1903.
[6] Selected examples:(a) Esteruelas, M. A.; Gómez, A. V.; López, A. M.; Oñate, E.; Ruiz, N. Organometallics 1999, 18, 1606.
(b) Bernad, D. J.; Esteruelas, M.; López, A. M.; Oliván, M.; Oñate, E.; Puerta, M. C.; Valerga, P. Organometallics 2000, 19, 4327.
(c) Kanao, K.; Tanabe, Y.; Miyake, Y.; Nishibayashi, Y. Organometallics 2010, 29, 2381.
(d) Queensen, M. J.; Rath, N. P.; Bauer, E. B. Organometallics 2014, 33, 5052.
(e) Strinitz, F.; Tucher, J.; Januszewski, J. A.; Waterloo, A. R.; Stegner, P.; Förtsch, S.; Hübner, E.; Tykwinski, R. R.; Burzlaff, N. Organometallics 2014, 33, 5129.
(f) Garcia de la Arada, I.; Díez, J.; Gamasa, M. P.; Lastra, E. J. Organomet. Chem. 2015, 797, 101.
[7] (a) Chen, K.-H.; Feng, Y. J.; Ma, H.-W.; Lin, Y.-C.; Liu, Y.-H.; Kuo, T.-S. Organometallics 2010, 29, 6829.
(b) Cadierno, V.; Conejero, S.; Gamasa, M. P.; Gimeno, J.; Falvello, L. R.; Llusar, R. M. Organometallics 2002, 21, 3716.
(c) Cadierno, V.; Gamasa, M. P.; Gimeno, J. Organometallics 1998, 17, 5216.
[8] (a) Serrano-Ruiz, M.; Lidrissi, C.; Mañas, S.; Peruzzini, M.; Romerosa, A. J. Organomet. Chem. 2014, 751, 654.
(b) Talavera, M.; Bolańo, S.; Bravo, J.; Castro, J.; García-Fontán, S.; Hermida-Ramón, J. M. Organometallics 2013, 32, 4402.
[9] (a) Venâncio, A. I. F. M.; Guedes da Silva, F. C.; Martins, L. M. D. R. S.; Fraústo da Silva, J. J. R.; Pombeiro, A. J. L. Organometallics 2005, 24, 4654.
(b) Cadierno, V.; Gamasa, M. P.; Gimeno, J.; López-González, M. C.; Borge, J.; Garciá-Granda, S. Organometallics 1997, 16, 4453.
[10] (a) Fischer, H.; Reindl, D.; Troll, C.; Leroux, F. J. Organomet. Chem. 1995, 490, 221.
(b) Esteruelas, M. A.; Gómez, A. V.; López, A. M.; Modriego, J.; Oñate, E. Organometallics 1998, 17, 5434.
(c) Bolańo, S.; Rodríguez-Rocha, M. M.; Bravo, J.; Castro, J.; Oñate, E.; Peruzzini, M. Organometallics 2009, 28, 6020.
(d) Jiménez-Tenorio, J.; Palacios, M. D.; Puerta, M. C.; Valerga, P. J. Organomet. Chem. 2004, 689, 2776.
(e) Peruzzini, M.; Barbaro, P.; Bertolasi, V.; Bianchini, C.; Mantovani, N.; Marvelli, L.; Rossi, R. Dalton Trans. 2003, 4121.
(f) Coletti, C.; Gonsalvi, L.; Guerriero, A.; Marvelli, L.; Peruzzini, M.; Reginato, G.; Re, N. Organometallics 2010, 29, 5982.
[11] Utegenov, K. I.; Krivykh, V. V. Glukhov, I. V. Petrovskii, P. V.; Ustynyuk, N. A. J. Organomet. Chem. 2011, 696, 3408.
[12] (a) Beletskaya, I. P.; Cheprakov, A. V. Organometallics 2012, 31, 7753.
(b) Hoover, J. M.; DiPasquale, A.; Mayer, J. M.; Michael, F. E. J. Am. Chem. Soc. 2010, 132, 5043.
(c) Schweizer, P. D.; Wadepohl, H.; Gade, L. H. Organometallics 2013, 32, 3697.
[13] (a) Barrett, A. G. M.; Carpenter, N. E.; Sabat, M. J. Organomet. Chem. 1988, 352, C8.
(b) Alt, H. G.; Engelhardt, H. E.; Steinlein, E.; Rogers, D. J. Organomet. Chem. 1987, 344, 321.
(c) Albertin, G.; Antoniutti, S.; Bortoluzzi, M.; Botter, A.; Castro, J. Dalton Trans. 2015, 44, 3439.
[14] Dabb, S. L.; Messerle, B. A.; Wagler, J. Organometallics 2008, 27, 4657.
[15] (a) Fukumoto, Y.; Dohi, T.; Masaoka, H.; Chatani, N.; Murai, S. Organometallics 2002, 21, 3845.
(b) Fukumoto, Y.; Tamura, Y.; Iyori, Y.; Chatani, N. J. Org. Chem. 2016, 81, 3161.
(c) Fukumoto, Y.; Ohmae, A.; Hirano, M.; Chatani, N. Asian J. Org. Chem. 2013, 2, 1036.
(d) Fukumoto, Y.; Asai, H.; Shimizu, M.; Chatani, N. J. Am. Chem. Soc. 2007, 129, 13792.
[16] Szesni, N.; Hohberger, C.; Mohamed, G. G.; Burzlaff, N.; Weibert, B.; Fischer, H. J. Organomet. Chem. 2006, 691, 5753.
[17] Cai, T.; Yang, Y.; Li, W.-W.; Qin, W.-B.; Wen, T.-B. Chem.-Eur. J. 2018, 24, 1606.
[18] Selegue, J. P. Organometallics 1982, 1, 217.
[19] (a) Aumann, R.; Jasper, B.; Fröhlich, R. Organometallics 1996, 14, 2447.
(b) Das, U. K.; Bhattacharjee, M. Chem.-Eur. J. 2012, 18, 5180.
(c) Sgro, M. J.; Stephan, D. W. Dalton Trans. 2013, 42, 10460.
[20] (a) Anil Kumar, P. G.; Pregosin, P. S.; Vallet, M.; Bernardinelli, G.; Jazzar, R. F.; Viton, F.; Kündig, E. P. Organometallics 2004, 23, 5410.
(b) Chiririwa, H.; Meijboom, R. Acta Crystallogr. 2011, E67, m1335.
[21] (a) Kopf, H.; Holzberger, B.; Pietraszuk, C.; Hübner, E.; Burzlaff, N. Organometallics 2008, 27, 5894.
(b) Jiménez-Tenorio, M.; Palacios, M. D.; Puerta, M. C.; Valerga, P. J. Organomet. Chem. 2004, 689, 2776.
(c) Bernad, D. J.; Esteruelas, M.; López, A. M.; Modrego, J.; Puerta, M. C.; Valerga, P. Organometallics 1999, 18, 4995.
[22] In the catalytic reactions, benzophenone or 9H-fluoren-9-one was also isolated as the byproducts (ca. 25%). We initially envisioned that the H2O presented in the reaction solution, which released along with the Selegue's reaction during the formation of the allenylidene complex, might lead to the hydrolysis of the acrylonitrile product 5 to give the respective ketones. We have performed the control experiments by heating a solution of 3,3-diphenylacrylo-nitrile (5a) in DCE with purposely added water at even 110℃ for several hours with or without 2.5 mol% of complex 1. However, the hydrolysis issue is unlikely. As reflected by the TLC of the reaction solution, only trace amount of benzophenone can be detected. On the other hand, it has been reported that γ-substituted tert-propargyl alcohols have been involved in Sonogashira-type reactions as masked terminal alkynes via β-carbon elimination with liberation of ketone (see Ref.
[23] ). We tentatively envisioned that the ketone byproducts obtained in the catalytic reaction might come from β-carbon elimination of terminal tert-propargyl alcohols.
[23] (a) Nishimura, T.; Ariki, H.; Maeda, Y.; Uemura, S. Org. Lett. 2003, 5, 2997.
(b) Funayama, A.; Satoh, T.; Miura, M. J. Am. Chem. Soc. 2005, 127, 15354.
(c) Li, T.; Wang, Z.; Zhang, M. L.; Zhang H.-J.; Wen, T.-B. Chem. Commun. 2015, 51, 6777.
(d) Li, T.; Wang, Z.; Qin, W.-B.; Wen, T.-B. ChemCatChem 2016, 8, 2146.
[24] Hao, L.; Wu, F.; Ding, Z.-C.; Xu, S.-X.; Ma, Y.-L.; Chen, L.; Zhan, Z.-P. Chem.-Eur. J. 2012, 18, 6453.
[25] Chiarucci, M.; Mocci, R.; Syntrivanis, L. D.; Cera, G.; Mazzanti, A.; Bandini, M. Angew. Chem., Int. Ed. 2013, 52, 10850.
[26] Hu, M. Y.; Ni, C. F.; Li, L. C.; Han, Y. X.; Hu, J. B. J. Am. Chem. Soc. 2015, 137, 14496.
[27] Shipilovskikh, S. A.; Vaganov, V. Y.; Denisova, E. I.; Rubtsov, A. E.; Malkov, A. V. Org. Lett. 2018, 20, 728.
/
| 〈 |
|
〉 |