

含苯并噻唑的硫脲嘧啶衍生物的合成及抗菌活性评价
收稿日期: 2018-01-27
修回日期: 2018-03-19
网络出版日期: 2018-06-01
基金资助
河北农业大学理工基金(No.LG20150503)资助项目.
Synthesis and Antibacterial Activity Evaluation of the Thiouracil Derivatives Containing Benzothiazole
Received date: 2018-01-27
Revised date: 2018-03-19
Online published: 2018-06-01
Supported by
Project supported by the Science and Technology Fund of Agricultural University of Hebei (No. LG20150503)
崔朋雷 , 刘娜 , 陈华 , 李小六 . 含苯并噻唑的硫脲嘧啶衍生物的合成及抗菌活性评价[J]. 有机化学, 2018 , 38(10) : 2784 -2790 . DOI: 10.6023/cjoc201801040
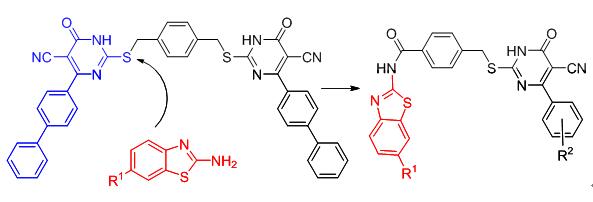
A series of unreported thiouracil derivatives 7 containing a benzothiazole moiety were synthesized by the reaction of 4-oxo-6-phenyl-2-thioxo-1,2,3,4-tetrahydro-pyrimidine-5-carbonitrile (6) with N-(benzo[d]thiazol-2-yl)-4-(chloromethyl)-benzamide (5), which was prepared from the amidation of 2-aminobenzothiazole and 4-(chloromethyl)benzoyl chloride. The newly synthesized compounds 7 were characterized by IR, MS, NMR, and element analysis. All the compounds were evaluated for their antibacterial activities against Bacillus amyloliquefaciens, Staphylococcus aureus and Bacillus subtilis. The results of the 24-hour antibacterial test showed that some compounds had good inhibitory activities against the three tested strains.
Key words: thiouracil; benzothiazole; antibacterial activity
[1] Davies, J.; Davies, D. Microbiol. Mol. Biol. Rev. 2010, 74, 417.
[2] Kumarasamy, K. K.; Toleman, M. A.; Walsh, T. R. Lancet Infect. Dis. 2010, 10, 597.
[3] Guillemot, D. Curr. Opin. Microbiol. 1999, 2, 494.
[4] Li, M. Y.; Huang, Y. J.; Tai, P. C.; Wang, B. H. Biochem. Biophys. Res. Commun. 2008, 368, 839.
[5] Chen, W. X.; Huang, Y. J.; Gundala, S. R.; Yang, H.; Li, M. Y.; Tai, P. C.; Wang, B. H. Bioorg. Med. Chem. 2010, 18, 1617.
[6] Chaudhary, A. S.; Jin, J. S.; Chen, W. X.; Tai, P. C.; Wang, B. H. Bioorg. Med. Chem. 2015, 23, 105.
[7] Cui, J. M.; Jin, J. S.; Chaudhary, A. S.; Hsieh, Y. Zhang, H.; Dai, C. F.; Damera, K.; Chen, W. X.; Tai, P. C.; Wang, B. H. ChemMed-Chem 2016, 11, 43.
[8] Cui, P. L.; Li, X. L.; Zhu, M. Y.; Wang, B. H.; Liu, J.; Chen, H. Bioorg. Med. Chem. Lett. 2017, 27, 2234.
[9] Cui, P. L.; Li, X. L.; Zhu, M. Y.; Wang, B. H.; Liu, J.; Chen, H. Eur. J. Med. Chem. 2017, 127, 159.
[10] Jiang, K.; Cao, L.; Hao, Z. F.; Chen, M. Y.; Chen, J. L.; Li, X.; Xiao, P.; Chen, L.; Wang, C. Y. Chin. J. Org. Chem. 2017, 37, 2221(in Chinese). (蒋凯, 曹梁, 郝志峰, 陈美燕, 程洁銮, 李晓, 肖萍, 陈亮, 汪朝阳, 有机化学, 2017, 37, 2221.)
[11] Zhang, Z. Y.; Li, W. J.; Ye, K. Q.; Zhang, H. Y. Acta Chim. Sinica 2016, 74, 179(in Chinese). (张振宇, 李婉君, 叶开其, 张红雨, 化学学报, 2016, 74, 179.)
[12] Moustafa, T. G.; Nadia, S. E. G.; Eman, R. E. B.; Mohamed, M. E. K.; Nanting, N. Chin. Chem. Lett. 2016, 27, 380.
[13] Abdou, W. M.; Barghash, R. F.; Sediek, A. A. Eur. J. Med. Chem. 2012, 57, 362.
[14] Yang, L. O.; Huang, Y. H.; Zhao, Y. W.; He, G.; Xie, Y. M.; Liu, J.; He, J.; Liu, B.; Wei, Y. Q. Bioorg. Med. Chem. Lett. 2012, 22, 3044.
[15] Suresh, M.; Sridevi, G.; Nuthangi, S.; Palakondu, L.; Sreekanth, B. J. Arabian J. Chem. 2016, 9, 681.
[16] Alessandro, S.; Kenneth, S.; Steven, D. J.; Bart, V.; Jef, R.; Jozef, A.; Piet, H. Chem. Biodiversity 2011, 8, 253.
[17] Mitra, R.; Mehdi, P.; Abolghasem, D.; Sattar, S. Chem. Heterocycl. Comd. 2015, 51, 918.
[18] Priya, R. M.; Chhaganbhai, N. P. Org. Med. Chem. Lett. 2012, 2, 29.
/
| 〈 |
|
〉 |