

钌催化β-酮酸酯不对称氢化反应的研究进展
收稿日期: 2018-02-24
修回日期: 2018-05-15
网络出版日期: 2018-06-07
基金资助
国家自然科学基金(Nos.21172081,21372090)、广东省自然科学基金重点(No.S2013020013091)和广州市科技计划(No.201510010054)资助项目.
Progress in Ruthenium-Catalyzed Asymmetric Hydrogenation of β-Keto Esters
Received date: 2018-02-24
Revised date: 2018-05-15
Online published: 2018-06-07
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21172081, 21372090), the Natural Science Foundation of Guangdong Province (No. S2013020013091) and the City of Guangzhou Science and Technology Plan Projects (No. 156300018).
陈姝琪 , 杨文 , 姚永祺 , 杨新 , 邓颖颍 , 杨定乔 . 钌催化β-酮酸酯不对称氢化反应的研究进展[J]. 有机化学, 2018 , 38(10) : 2534 -2552 . DOI: 10.6023/cjoc201802022
Ruthenium-catalyzed asymmetric hydrogenation of β-keto esters is one of the most effective methods for the synthesis of chiral β-hydroxy esters. The recent research progress in ruthenium-catalyzed asymmetric hydrogenation of β-keto esters is reviewed. Great attention was paid to the influences of chiral ligands, substrate structure, solvents and additives on the homogeneous asymmetric hydrogenation, as well as the influences of support materials and additives on the heterogeneous asymmetric hydrogenation.
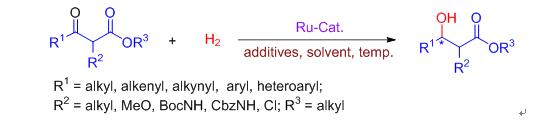
[1] Parshall, G. W.; Nugent, W. A. CHEMTECH 1988, 18, 184.
[2] Parshall, G. W.; Nugent, W. A. CHEMTECH 1988, 18, 376.
[3] Yin, Y.-Q.; Jiang, Y.-Z. Progress in Asymmetric Catalytic Reactions, Science Press, Beijing, 2000, p. 13(in Chinese). (殷元骐, 蒋耀忠, 不对称催化反应进展, 科学出版社, 北京, 2000, p. 13.)
[4] Knowles, W. S.; Sabacky, M. J. Chem. Commun. 1968, 72, 1445.
[5] Miyashita, A.; Yasuda, A.; Takaya, H.; Toriumi, K.; Ito, T.; Souchi, T.; Noyori, R. J. Am. Chem. Soc. 1980, 102, 7932.
[6] Li, S.-S. Chin. J. Nat. 2001, 23, 349(in Chinese). (李声时, 自然杂志, 2001, 23, 349.)
[7] (a) Chen, Q.-A.; Gao, K.; Duan, Y.; Ye, Z.-S.; Shi, L.; Yang, Y.; Zhou, Y.-G. J. Am. Chem. Soc. 2012, 134, 2442.
(b) Li, J.; Shen, J.-F.; Xia, C.; Wang, Y.-Z.; Liu, D.-L.; Zhang, W.-B. Org. Lett. 2016, 18, 2122.
[8] (a) Jiang, J.; Lu, W.-X.; Lv, H.; Zhang, X.-M. Org. Lett. 2015, 17, 1154.
(b) Li, P.; Zhou, M.; Zhao, Q.-Y.; Wu, W.-L.; Hu, X.-Q.; Dong X.-Q.; Zhang, X.-M. Org. Lett. 2016, 18, 40.
[9] (a) Wu, W.-L.; Liu, S.-D.; Duan, M.; Tan, X.-F.; Chen, C.-Y.; Xie, Y.; Lan, Y.; Dong, X.-Q.; Zhang, X.-M. Org. Lett. 2016, 18, 2938.
(b) Wu. W.-L.; You, C.; Yin, C.-C.; Liu, Y.-H.; Dong, X.-Q.; Zhang, X.-M. Org. Lett. 2017, 19, 2548.
(c) Deng, Y.-Y.; Yang, W.; Yang, X.; Yang, D.-Q. Chin. J. Org. Chem. 2017, 37, 3039(in Chinese). (邓颖颍, 杨文, 杨新, 杨定乔, 有机化学, 2017, 37, 3039.)
[10] (a) Mondelli, C.; Vargas, A.; Santarossa, G.; Baiker, A. J. Phys. Chem. C 2009, 113, 15246.
(b) Meemken, F.; Baiker, A.; Dupré, J.; Hungerbühler, K. ACS Catal. 2014, 4, 344.
(c) Guan, S.-L.; Donovan-Sheppard, O.; Reece, C.; Willock, D. J.; Wain, A. J.; Attard, G. A. ACS Catal. 2016, 6, 1822.
[11] (a) Chen, M.-W.; Duan, Y.; Chen, Q.-A.; Wang, D.-S.; Yu, C.-B.; Zhou, Y.-G. Org. Lett. 2010, 12, 5075.
(b) Chen, Z.-P.; Hu, S.-B.; Zhou, J.; Zhou, Y.-G. ACS Catal. 2015, 5, 6086.
[12] Lv, M.; Liu, H.-W. Guangdong Chem. Ind. 2014, 41, 150(in Chinese). (吕明, 刘华伟, 广东化工, 2014, 41, 150.)
[13] Paul, D.; Beiring, B.; Plois, M.; Ortega, N.; Kock, S.; Schlüns, D.; Neugebauer, J.; Wolf, R.; Glorius, F. Organometallics 2016, 35, 3641.
[14] Eichenseer, C. M.; Kastl, B.; Pericàs, M. A.; Hanson, P. R.; Reiser, O. ACS Sustainable Chem. Eng. 2016, 4, 2698.
[15] Ding, Z.-Y.; Chen, F.; Qin, J.; He, Y.-M.; Fan, Q.-H. Angew. Chem., Int. Ed. 2012, 51, 5706.
[16] (a) Xie, J.-H.; Zhou, Q.-L. Acta Chim. Sinica 2014, 72, 778(in Chinese). (谢建华, 周其林, 化学学报, 2014, 72, 778.)
(b) Yuan, Q.-J.; Zhang, W.-B. Chin. J. Org. Chem. 2016, 36, 274(in Chinese). (袁乾家, 张万斌, 有机化学, 2016, 36, 274.)
[17] Guo, H.-C.; Ding, K.-L.; Dai, L.-X. Chin. Sci. Bull. 2004, 49, 1575(in Chinese). (郭红超, 丁奎岭, 戴立信, 科学通报, 2004, 49, 1575.)
[18] Claver, C.; Fernandez, E.; Gillon, A.; Heslop, K.; Hyett, D. J.; Martorell, A.; Orpen, A. G.; Pringle, P. G. Chem. Commun. 2000, 961.
[19] Öchsner, E.; Etzold, B.; Junge, K.; Beller, M.; Wasserscheid, P. Adv. Synth. Catal. 2009, 351, 235.
[20] Dong, J.-X.; Yang, D.-Q.; Liu, E.-C.; Jiang, K.-L.; Han, Y.-F. Chem. Ind. Times 2005, 19, 40(in Chinese). (董建霞, 杨定乔, 刘二畅, 姜凯龄, 韩英峰, 化工时刊, 2005, 19, 40.)
[21] Korostylev, A.; Andrushko, V.; Andrushko, N.; Tararov, V. I.; König, G.; Börner, A. Eur. J. Org. Chem. 2008, 840.
[22] Hamada, Y.; Makino, K. J. Synth. Org. Chem., Jpn. 2008, 66, 1057.
[23] Makino, K.; Goto, T.; Hiroki, Y.; Hamada, Y. Tetrahedron:Asymmetry 2008, 19, 2816.
[24] Makino, K.; Goto, T.; Ohtaka, J.; Hamada, Y. Heterocycles 2009, 77, 629.
[25] Roche, C.; Desroy, N.; Haddad, M.; Phansavath, P.; Genet, J. P. Org. Lett. 2008, 10, 3911.
[26] Roche, C.; Roux, R. L.; Haddad, M.; Phansavath, P.; Genet, J. P. Synlett 2009, 573.
[27] Prévost, S.; Gauthier, S.; De Andrade, M. C.; Mordant, C.; Touati, A. R.; Lesot, P.; Savignac, P.; Ayad, T.; Phansavath, P.; Ratovelomanana-Vidal, V.; Genet, J. P. Tetrahedron:Asymmetry 2010, 21, 1436.
[28] Prévost, S.; Ayad, T.; Phansavath, P.; Ratovelomanana-Vidal, V. Adv. Synth. Catal. 2011, 353, 3213.
[29] Touati, R.; Hassine, B. B. J. Soc. Chim. Tunis. 2008, 10, 127.
[30] Kesselgruber, M.; Lotz, M.; Martin, P.; Melone, G.; Müller, M.; Pugin, B.; Naud, F.; Spindler, F.; Thommen, M.; Zbinden, P.; Blaser, H. U. Chem. Asian J. 2008, 3, 1384.
[31] Chen, L.; Ma, M.-L.; Peng, Z.-H.; Chen, H. Chin. J. Org. Chem. 2008, 28, 1724(in Chinese). (陈丽, 马梦林, 彭宗海, 陈华, 有机化学, 2008, 28, 1724.)
[32] Peng, Z.-H.; Ma, M.-L.; Fu, H.-Y.; Chen, H.; Li, X.-J. Chin. J. Catal. 2010, 31, 191(in Chinese). (彭宗海, 马梦林, 付海燕, 陈华, 李贤钧, 催化学报, 2010, 31, 191.)
[33] Sun, X.-F.; Li, W.; Hou, G.-H.; Zhou, L.; Zhang, X.-M. Adv. Synth. Catal. 2009, 351, 2553.
[34] Wang, C.-J.; Wang, C.-B.; Chen, D.; Yang, G.-F.; Wu, Z.-S.; Zhang, X.-M. Tetrahedron Lett. 2009, 50, 1038.
[35] Zhang, Z.; Qian, H.; Longmire, J.; Zhang, X. J. Org. Chem. 2000, 65, 6223.
[36] Sun, X.-F.; Li, W.; Zhou, L.; Zhang, X.-M. Chem.-Eur. J. 2009, 15, 7302.
[37] Yuan, W.-C.; Cun, L.-F.; Mi, A.-Q.; Jiang, Y.-Z.; Gong, L.-Z. Tetrahedron 2009, 65, 4130.
[38] Oki, H.; Oura, I.; Nakamura, T.; Ogata, K.; Fukuzawa, S. I. Tetrahedron:Asymmetry 2009, 20, 2185.
[39] Floris, T.; Kluson, P.; Slater, M. React. Kinet., Mech. Catal. 2011, 102, 67.
[40] Yao, Y.; Fan, W.-Z.; Li, W.-F.; Ma, X.; Zhu, L.-F.; Xie, X.-M.; Zhang, Z.-G. J. Org. Chem. 2011, 76, 2807.
[41] Fan, W.-Z.; Li, W.-F.; Ma, X.; Tao, X.-M.; Li, X.-M.; Yao, Y.; Xie, X.-M.; Zhang, Z.-G. J. Org. Chem. 2011, 76, 9444.
[42] Li, W.-F.; Ma, X.; Fan, W.-Z.; Tao, X.-M.; Li, X.-M.; Xie, X.-M.; Zhang, Z.-G. Org. Lett. 2011, 13, 3876.
[43] Fan, W.-Z.; Li, W.-F.; Ma, X.; Tao, X.-M.; Li, X.-M.; Yao, Y.; Xie, X.-M.; Zhang, Z.-G. Chem. Commun. 2012, 48, 4247.
[44] Ma, X.; Li, W.-F.; Li, X.-M.; Tao, X.-M.; Fan, W.-Z.; Xie, X.-M.; Ayad, T.; Ratovelomanana-Vidal, V.; Zhang, Z.-G. Chem. Commun. 2012, 48, 5352.
[45] Li, W.-F.; Tao, X.-M.; Ma, X.; Fan, W.-Z.; Li, X.-M.; Zhao, M.-M.; Xie, X.-M.; Zhang, Z.-G. Chem.-Eur. J. 2012, 18, 16531.
[46] Li, X.-M.; Tao, X.-M.; Ma, X.; Li, W.-F.; Zhao, M.-M.; Xie, X.-M.; Ayad, T.; Ratovelomanana-Vidal, V.; Zhang, Z.-G. Tetrahedron 2013, 69, 7152.
[47] Satyanarayana, P.; Maheswaran, H.; Kantama, M. L.; Chawla, H. P. S. Catal. Sci. Technol. 2012, 2, 2508.
[48] Brewitz, L.; Llaveria, J.; Yada, A.; Fürstner, A. Chem.-Eur. J. 2013, 19, 4532.
[49] Haydl, A. M.; Breit, B. Chem.-Eur. J. 2017, 23, 541.
[50] Cartigny, D.; Püntener, K.; Ayad, T.; Scalone, M.; Ratovelomanana-Vidal, V. Org. Lett. 2010, 12, 3788.
[51] Monnereau, L.; Cartigny, D.; Scalone, M.; Ayad, T.; Ratovelomanana-Vida, V. Chem.-Eur. J. 2015, 21, 11799.
[52] Echeverria, P. G.; Cornil, J.; Férard, C.; Guérinot, A.; Cossy, J.; Phansavath, P.; Ratovelomanana-Vidal, V. RSC Adv. 2015, 5, 56815.
[53] Perez, M.; Echeverria, P. G.; Martinez-Arripe, E.; Zoubir, M. E.; Touati, R.; Zhang, Z.-G.; Genet, J. P.; Phansavath, P.; Ayad, T.; Ratovelomanana-Vidal, V. Eur. J. Org. Chem. 2015, 5949.
[54] Liu, Z.-Q.; Shultz, C. S.; Sherwood, C. A.; Krska, S.; Dormer, P. G.; Desmond, R.; Lee, C.; Sherer, E. C.; Shpungin, J.; Cuff, J.; Xu, F. Tetrahedron Lett. 2011, 52, 1685.
[55] Fang, Z.-J.; Wills, M. J. Org. Chem. 2013, 78, 8594.
[56] Kisic, A.; Stephan, M.; Mohar, B. Org. Lett. 2013, 15, 1614.
[57] Rast, S.; Modec, B.; Stephan, M.; Mohar, B. Org. Biomol. Chem. 2016, 14, 2112.
[58] Sun, G.-D.; Zhou, Z.-H.; Luo, Z.-H.; Wang, H.-L.; Chen, L.; Xu, Y.-B.; Li, S.; Jian, W.-L.; Zeng, J.-B.; Hu, B.-Q.; Han, X.-D.; Lin, Y.-C.; Wang, Z.-Q. Org. Lett. 2017, 19, 4339.
[59] Weng, W.; Zhou, H.-Y.; Fu, H.-X.; Lv, S.-J. Chin. J. Org. Chem. 1998, 18, 509(in Chinese). (翁文, 周宏英, 傅宏祥, 吕士杰, 有机化学, 1998, 18, 509.)
[60] Seashore-Ludlow, B.; Villo, P.; Häcker, C.; Somfai, P. Org. Lett. 2010, 12, 5274.
[61] Wang, X.-L.; Xu, L.-J.; Yan, L.-J.; Wang, H.-F.; Han, S.; Wu, Y.; Chen, F.-E. Tetrahedron 2016, 72, 1787.
[62] Yamamura, T.; Nakatsuka, H.; Tanaka, S.; Kitamura, M. Angew. Chem. 2013, 125, 9483.
[63] Yamamura, T.; Nakane, S.; Nomura, Y.; Tanaka, S.; Kitamura, M. Tetrahedron 2016, 72, 3781.
[64] Lipkin, D.; Stewart, T. D. J. Am. Chem. Soc. 1939, 61, 3297.
[65] Zuo, X.-B.; Liu, H.-F. J. Mol. Catal. 1997, 11, 309(in Chinese). (左晓斌, 刘汉范, 分子催化, 1997, 11, 309.)
[66] Lu, F.-S.; Chao, J.-P.; Yang, C.-Y. Prog. Chem. 2001, 13, 192(in Chinese). (鲁福身, 晁建平, 杨春育, 化学进展, 2001, 13, 192.)
[67] Song, C. E. Annu. Rep. Prog. Chem., Sect. C:Phys. Chem. 2005, 101, 143.
[68] McDonald, A. R.; Müller, C.; Vogt, D.; Klinka, G. P. M. V.; Koten, G. V. Green Chem. 2008, 10, 424.
[69] Ahn, S. H.; Choi, M. S.; Im, J. S.; Sheikh, R.; Park, Y. H. J. Mol. Catal. A:Chem. 2013, 373, 55.
[70] Kluson, P.; Krystynik, P.; Dytrych, P.; Bartek, L. React. Kinet., Mech. Cat. 2016, 119, 393.
[71] Dongil, A. B. Bachiller-Baeza, B.; Guerrero-Ruiz, A.; Rodríguez-Ramos, I. Catal. Commun. 2012, 26, 149.
[72] Seki, T.; McEleney, K.; Crudden, C. M. Chem. Commun. 2012, 48, 6369.
[73] Peng, J.; Wang, X.-F.; Zhang, X.-M.; Bai, S.-Y.; Zhao, Y.-P.; Li, C.; Yang, Q.-H. Catal. Sci. Technol. 2015, 5, 666.
[74] Sun, Q.; Meng, X.-J.; Liu, X.; Zhang, X.-M.; Yang, Y.; Yang, Q.-H.; Xiao, F.-S. Chem. Commun. 2012, 48, 10505.
[75] Wang, X.; Lu, S.-M.; Li, J.; Liu, Y.; Li, C. Catal. Sci. Technol. 2015, 5, 2585.
[76] Wang, T.; Lyu, Y.; Xiong, K.; Wang, W.-L.; Zhang, H.; Zhan, Z.-P.; Jiang, Z.; Ding, Y.-J. Chin. J. Catal. 2017, 38, 890.
[77] Kong, S.-N.; Malik, A. U.; Qian, X.-F.; Shu, M.-H.; Xiao, W.-D. Chin. J. Org. Chem. 2018, 38, 656(in Chinese). (孔胜男, Abaid Ullah Malik, 钱雪峰, 舒谋海, 肖文德, 有机化学, 2018, 38, 656.)
[78] Falkowski, J. M.; Sawano, T.; Zhang, T.; Tsun, G.; Chen, Y.; Lockard, J. V.; Lin, W.-B. J. Am. Chem. Soc. 2014, 136, 5213.
[79] Wang, W.-W.; Wang, Q.-R. Chem. Commun. 2010, 46, 4616.
[80] Wang, W.-W.; Li, Z.-M.; Su, L.; Wang, Q.-R.; Wu, Y.-L. J. Mol. Catal., A 2014, 387, 92.
[81] Ma, B.-D.; Miao, T.-T.; Sun, Y.-H.; He, Y.-M.; Liu, J.; Feng, Y.; Chen, H.; Fan, Q.-H. Chem.-Eur. J. 2014, 20, 9969.
[82] Wang, W.-W.; Li, Z.-M.; Mu, W.-B.; Su, L.; Wang, Q.-R. Catal. Commun. 2010, 11, 480.
[83] Zeror, S.; Collin, J.; Fiaud, J. C.; Zouioueche, L. A. Tetrahedron:Asymmetry 2010, 21, 1211.
[84] Seashore-Ludlow, B.; Villo, P.; Somfai, P. Chem.-Eur. J. 2012, 18, 7219.
[85] Seashore-Ludlow, B. Saint-Dizier, F.; Somfai, P. Org. Lett. 2012, 14, 6334.
[86] Floris, T.; Kluson, P.; Bartek, L.; Pelantova, H. Appl. Catal., A 2009, 366, 160.
[87] Öchsner, E.; Schneiders, K.; Junge, K.; Beller, M.; Wasserscheid, P. Appl. Catal., A 2009, 364, 8.
[88] Öchsner, E.; Schneider, M. J.; Meyer, C.; Haumann, M.; Wasserscheid, P. Appl. Catal., A 2011, 399, 35.
[89] Jin, X.; Kong, F.-F.; Yang, Z.-Q.; Cui, F.-F. J. Mol. Catal. A-Chem. 2013, 374, 22.
[90] Turova, O. V.; Kuchurov, I. V.; Starodubtseva, E. V.; Ferapontov, V. A.; Ikonnikov, N. S.; Zlotina, S. G.; Vinogradov, M. G. Mendeleev Commun. 2012, 22, 184.
/
| 〈 |
|
〉 |