

天然产物Arborisidine四环骨架的不对称合成
收稿日期: 2018-05-10
修回日期: 2018-06-04
网络出版日期: 2018-06-15
基金资助
国家自然科学基金(Nos.21572140,21732005)资助项目.
Asymmetric Synthesis of the Tetracyclic Skeleton of Natural Product Arborisidine
Received date: 2018-05-10
Revised date: 2018-06-04
Online published: 2018-06-15
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21572140, 21732005).
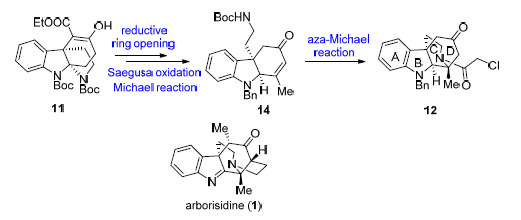
报道了吲哚生物碱arborisidine四环骨架的不对称合成研究.以本课题组前期报道的光学纯化合物10,9-二叔丁基-5-乙基(4S,8R)-7,8-二羟基-9H-8a,4b-(氨基乙醇氨基)-咔唑-5,9,10-三羧酸酯(11)为起始原料,经Krapcho反应脱羧转化为酮后,通过还原开环策略实现酮中间体四氢吡咯环的开环;随后经两次Saegusa氧化反应和Michael加成反应,实现了A/B/D三环中间C(15)-C(16)双键的构建和C(16)位甲基取代的引入.最后经分子内氮杂Michael加成反应一步实现arborisidine中哌啶C环和C(16)季碳中心的建立,高效完成目标分子A/B/C/D四环骨架的不对称合成.
关键词: 吲哚生物碱; arborisidine; Saegusa氧化; 氮杂Michael加成; 不对称合成
陈志涛 , 肖涛 , 宋颢 , 秦勇 . 天然产物Arborisidine四环骨架的不对称合成[J]. 有机化学, 2018 , 38(9) : 2427 -2434 . DOI: 10.6023/cjoc201805025
Asymmetric synthesis of the tetracyclic skeleton of arborisidine is reported. Starting from the enantiomerically pure compound 10,9-di-tert-butyl5-ethyl(4S,8R)-6-hydroxy-7,8-dihydro-9H-8a,4b-(epiminoethano)carbazole-5,9,10-tricar-boxylate (11) which was reported earlier by our group, a Krapcho decarboxylation reaction was used to afford the ketone, and then a reductive ring-opening method was applied to open the pyrrolidine ring of the substrate. The C(15)-C(16) double bond and the methyl group at C(16) of A/B/D tricyclic skeleton were introduced via Saegusa oxidation and Michael reaction, respectively. Finally, an intramolecular aza-Michael addition reaction was used as a key reaction to construct the C-ring and C(16) quaternary center, which led to the efficiently asymmetric synthesis of A/B/C/D tetracyclic skeleton of arborisidine.

[1] Wong, S.-P.; Chong, K.-W.; Lim, K.-H.; Lim, S.-H.; Low, Y.-Y.; Kam, T.-S. Org. Lett. 2016, 18, 1618.
[2] Wong, S.-P.; Gan, C.-Y.; Lim, K.-H.; Ting, K.-N.; Low, Y.-Y.; Kam, T.-S. Org. Lett. 2015, 17, 3628.
[3] Subramaniam, G.; Hiraku, O.; Hayashi, M.; Koyano, T.; Komiyama, K.; Kam, T.-S. J. Nat. Prod. 2007, 70, 1783.
[4] Schnoes, H. K.; Biemann, K.; Mokry, J.; Kompis, L.; Chatterjee, A.; Ganguli, G. J. Org. Chem. 1966, 31, 1641.
[5] Li, L.; Yang, T.; Liu, Y.; Liu, J.; Li, M.; Wang, Y.; Yang, S.; Zou, Q.; Li, G. Org. Lett. 2012, 14, 3450.
[6] Gan, P.; Pitzen, J.; Qu, P.; Snyder, S. A. J. Am. Chem. Soc. 2018, 140, 919.
[7] (a) Zu, L.; Boal, B. W.; Garg, N. K. J. Am. Chem. Soc. 2011, 133, 8877.
(b) Ren, W.; Wang, Q.; Zhu, J. Angew. Chem., Int. Ed. 2014, 53, 1818.
(c) Teng, M.; Zi, W.; Ma, D. Angew. Chem., Int. Ed. 2014, 53, 1814.
[8] (a) Moreno, J.; Picazo, E.; Morrill, L. A.; Smith, J. M.; Garg, N. K. J. Am. Chem. Soc. 2016, 138, 1162.
(b) Jiang, S.-Z.; Zeng, X.-Y.; Liang, X.; Lei, T.; Wei, K.; Yang, Y.-R. Angew. Chem., Int. Ed. 2016, 55, 4044.
(c) Wang, T.; Duan, X.; Zhao, H.; Zhai, S.; Tao, C.; Wang, H.; Li, Y.; Cheng, B.; Zhai, H. Org. Lett. 2017, 19, 1650.
[9] Moreno, J.; Picazo, E.; Morrill, L. A.; Smith, J. M.; Garg, N. K. J. Am. Chem. Soc. 2016, 138, 1162.
[10] Ren, W.; Wang, Q.; Zhu, J. Angew. Chem., Int. Ed. 2016, 55, 3500.
[11] (a) Xie, X.; Wei, B.; Li, G.; Zu, L. Org. Lett. 2017, 19, 5430.
(b) Li, G.; Xie, X.; Zu, L. Angew. Chem., Int. Ed. 2016, 55, 10483.
[12] Nishiyama, D.; Ohara, A.; Chiba, H.; Kumagai, H.; Oishi, S.; Fujii, N.; Ohno, H. Org. Lett. 2016, 18, 1670.
[13] (a) Eckermann, R.; Breunig, M.; Gaich, T. Chem. Commun. 2016, 52, 11363.
(b) Eckermann, R.; Breunig, M.; Gaich, T. Chem.-Eur. J. 2017, 23, 3938.
[14] Smith, M. W.; Zhou, Z.; Gao, A. X.; Shimbayashi, T.; Snyder, S. A. Org. Lett. 2017, 19, 1004.
[15] (a) Xiao, T.; Chen, Z.-T.; Deng, L.-F.; Zhang, D.; Liu, X.-Y.; Song, H.; Qin, Y. Chem. Commun. 2017, 53, 12665.
(b) Chen, Z.-T.; Xiao, T.; Tang, P.; Zhang, D.; Qin, Y. Tetrahedron 2018, 74, 1129.
[16] Zhang, D.; Song, H.; Qin, Y. Acc. Chem. Res. 2011, 44, 447
[17] Shen, L.; Zhang, M.; Wu, Y.; Qin, Y. Angew. Chem., Int. Ed. 2008, 47, 3618.
[18] Zhang, M.; Huang, X.; Shen, L.; Qin, Y. J. Am. Chem. Soc. 2009, 131, 6013.
[19] (a) Eckermann, R.; Gaich, T. Synthesis 2013, 45, 2813.
(b) Smith, J. M.; Moreno, J.; Boal, B. W.; Garg, N. K. Angew. Chem., Int. Ed. 2015, 54, 400.
(c) Adams, G, L; Smith, A. B. The Alkaloids. Chemistry and Biology, Elsevier, New York, 2016, 76, 171.
/
| 〈 |
|
〉 |