

手性传感器研究进展
收稿日期: 2018-05-20
修回日期: 2018-06-05
网络出版日期: 2018-06-22
基金资助
中国医学科学院医学与健康科技创新工程(No.2016-I2M-3-009)资助项目.
Advances in Development of Chiral Sensors
Received date: 2018-05-20
Revised date: 2018-06-05
Online published: 2018-06-22
Supported by
Project supported by the Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (No. 2016-I2M-3-009).
熊斐 , 李莉 . 手性传感器研究进展[J]. 有机化学, 2018 , 38(11) : 2927 -2936 . DOI: 10.6023/cjoc201805042
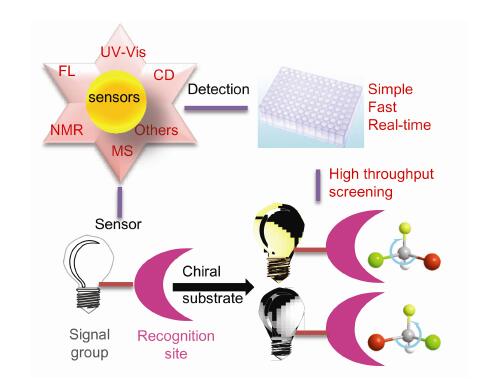
Chiral compounds play an essential role in asymmetric synthesis, biology, medical field and pharmacology. It is necessary to establish fast, sensitive and high enantioselective chiral analysis methods. Chiral sensors, which could determine the absolute configuration and the value of enantiomeric excess of enantiomers, have the advantages of simple, rapid, sensitive and real-time. Herein, the review is focused on the recent progress of chiral fluorescent sensors, circular dichroism sensors, UV-Vis sensors, NMR sensors and MS sensors. Their characteristics, sensing mechanism and applications in chiral recognition are reviewed, and the prospects of chiral sensors are also discussed.
[1] Wu, L.; Vogt, F. G. J. Pharm. Biomed. Anal. 2012, 69, 133.
[2] Yun, Y.; Gellman, A. J. Nat. Chem. 2015, 7, 520.
[3] Tang, S.; Bin, Q.; Chen, W.; Bai, Z.-W.; Huang, S.-H. J. Chromatogr. A 2016, 1440, 112.
[4] Zhang, J.-H.; Xie, S.-M.; Zhang, M.; Zi, M.; He, P.-G.; Yuan, L.-M. Anal. Chem. 2014, 86, 9595.
[5] Kalíková, K.; Šlechtová, T.; Vozka, J.; Tesarová, E. Anal. Chim. Acta 2014, 821, 1.
[6] He, J.; Wang, X.; Morill, M.; Shamsi, S. A. Anal. Chem. 2012, 84, 5236.
[7] Fortuna, A.; Alves, G.; Falcão, A. Biomed. Chromatogr. 2014, 28, 27.
[8] Shen, Z.; Lv, C.; Zeng, S. J. Pharm. Anal. 2016, 6, 1.
[9] Sun, W.; Guo, S.; Hu, C.; Fan, J.; Peng, X. Chem. Rev. 2016, 116, 7768.
[10] Zhang, X.; Yin, J.; Yoon, J. Chem. Rev. 2014, 114, 4918.
[11] You, L.; Zha, D.; Anslyn, E. V. Chem. Rev. 2015, 115, 7840.
[12] Li, Z.; Mo, Z.; Meng, S.; Gao, H.; Niu, X.; Guo, R. Anal. Methods 2016, 8, 8134.
[13] Trojanowicz, M. Electrochem. Commun. 2014, 38, 47.
[14] Zhou, X.; Lee, S.; Xu, Z.; Yoon, J. Chem. Rev. 2015, 115, 7944.
[15] Pu, L. Acc. Chem. Res. 2011, 45, 150.
[16] Chen, Z.; Wang, Q.; Wu, X.; Li, Z.; Jiang, Y.-B. Chem. Soc. Rev. 2015, 44, 4249.
[17] Wong, J. K.-H.; Todd, M. H.; Rutledge, P. J. Molecules 2017, 22, 200.
[18] De Silva, A. P.; Gunaratne, H. N.; Gunnlaugsson, T.; Huxley, A. J.; McCoy, C. P.; Rademacher, J. T.; Rice, T. E. Chem. Rev. 1997, 97, 1515.
[19] Pu, L. Chem. Rev. 2004, 104, 1687.
[20] Leung, D.; Kang, S. O.; Anslyn, E. V. Chem. Soc. Rev. 2012, 41, 448.
[21] Lu, Z.-Y.; Zhang, R.-K.; Li, G.-K. J. Instrum. Anal. 2017, 36, 570(in Chinese). (路振宇, 张润坤, 李攻科, 分析测试学报, 2017, 36, 570.)
[22] Qin, T.-Y.; Zeng, Y.; Chen, J.-P.; Yu, T.-J.; Li, Y. Acta Chim. Sinica 2017, 75, 1164(in Chinese). (秦天依, 曾毅, 陈金平, 于天君, 李嫕, 化学学报, 2017, 75, 1164.)
[23] Huang, Z.; Yu, S.; Zhao, X.; Wen, K.; Xu, Y.; Yu, X.; Xu, Y.; Pu, L. Chem.-Eur. J. 2014, 20, 16458.
[24] Yu, S.; Plunkett, W.; Kim, M.; Pu, L. J. Am. Chem. Soc. 2012, 134, 20282.
[25] Wen, K.; Yu, S.; Huang, Z.; Chen, L.; Xiao, M.; Yu, X.; Pu, L. J. Am. Chem. Soc. 2015, 137, 4517.
[26] Wang, C.; Wu, E.; Wu, X.; Xu, X.; Zhang, G.; Pu, L. J. Am. Chem. Soc. 2015, 137, 3747.
[27] Pu, L. Acc. Chem. Res. 2017, 50, 1032.
[28] Huang, Z.; Yu, S.; Wen, K.; Yu, X.; Pu, L. Chem. Sci. 2014, 5, 3457.
[29] Zhao, F.; Du, Y.; Tian, J.; Shi, D.; Wang, Y.; Hu, L.; Yu, S.; Yu, X.; Pu, L. Eur. J. Org. Chem. 2018, 2018, 1891.
[30] Zhou, Q.; Swager, T. M. J. Am. Chem. Soc. 1995, 117, 12593.
[31] Zhang, X.; Wang, C.; Wang, P.; Du, J.; Zhang, G.; Pu, L. Chem. Sci. 2016, 7, 3614.
[32] Song, F.; Wei, G.; Jiang, X.; Li, F.; Zhu, C.; Cheng, Y. Chem. Commun. 2013, 49, 5772.
[33] Xia, H.; Chen, X.; Chen, X.; Cheng, J.; Liu, Y.; Chen, X.; Zhang, Q.; Zou, G. Sens. Actuators, B 2018, 254, 44.
[34] Feng, H.-T.; Zhang, X.; Zheng, Y.-S. J. Org. Chem. 2015, 80, 8096.
[35] Akdeniz, A.; Minami, T.; Watanabe, S.; Yokoyama, M.; Ema, T.; Anzenbacher, P. Chem. Sci. 2016, 7, 2016.
[36] Wu, J.; Lu, J.; Liu, J.; Zheng, C.; Gao, Y.; Hu, J.; Ju, Y. Sens. Actuators, B 2017, 241, 931.
[37] Zeng, C.; Zhang, X.; Pu, L. Chem.-Eur. J. 2017, 23, 2432.
[38] Feagin, T. A.; Olsen, D. P.; Headman, Z. C.; Heemstra, J. M. J. Am. Chem. Soc. 2015, 137, 4198.
[39] Cai, P.; Wu, D.; Zhao, X.; Pan, Y. Analyst 2017, 142, 2961.
[40] Wanderley, M. M.; Wang, C.; Wu, C.-D.; Lin, W. J. Am. Chem. Soc. 2012, 134, 9050.
[41] Zhang, F.; Lu, C.; Wang, M.; Yu, X.; Wei, W.; Xia, Z. ACS Sens. 2018, 3, 304.
[42] Dai, L.; Wu, W.; Liang, W.; Chen, W.; Yu, X.; Ji, J.; Xiao, C.; Yang, C. Chem. Commun. 2018, 54, 2643.
[43] Yao, J.; Wu, W.; Liang, W.; Feng, Y.; Zhou, D.; Chruma, J. J.; Fukuhara, G.; Mori, T.; Inoue, Y.; Yang, C. Angew. Chem., Int. Ed. 2017, 56, 6869.
[44] Yang, C.; Inoue, Y. Chem. Soc. Rev. 2014, 43, 4123.
[45] Bentley, K. W.; Nam, Y. G.; Murphy, J. M.; Wolf, C. J. Am. Chem. Soc. 2013, 135, 18052.
[46] Zhang, P.; Wolf, C. Chem. Commun. 2013, 49, 7010.
[47] Bentley, K. W.; Wolf, C. J. Am. Chem. Soc. 2013, 135, 12200.
[48] Bentley, K. W.; Wolf, C. J. Org. Chem. 2014, 79, 6517.
[49] De, Z. L. S.; Ding, R.; Wolf, C. Org. Biomol. Chem. 2016, 14, 1934.
[50] Thanzeel, F. Y.; Wolf, C. Angew. Chem., Int. Ed. 2017, 56, 7276.
[51] Pilicer, S. L.; Bakhshi, P. R.; Bentley, K. W.; Wolf, C. J. Am. Chem. Soc. 2017, 139, 1758.
[52] Zhao, Y.-W.; Wang, Y.; Zhang, X.-M. ACS Appl. Mater. Interfaces 2017, 9, 20991.
[53] Seo, M.-S.; Sun, D.; Kim, H. J. Org. Chem. 2017, 82, 6586.
[54] Wang, L.-L.; Chen, Z.; Liu, W.-E.; Ke, H.; Wang, S.-H.; Jiang, W. J. Am. Chem. Soc. 2017, 139, 8436.
[55] Maeda, K.; Hirose, D.; Okoshi, N.; Shimomura, K.; Wada, Y.; Ikai, T.; Kanoh, S.; Yashima, E. J. Am. Chem. Soc. 2018, 140, 3270.
[56] Lu, W.; Yang, H.; Li, X.; Wang, C.; Zhan, X.; Qi, D.; Bian, Y.; Jiang, J. Inorg. Chem. 2017, 56, 8223.
[57] Anyika, M.; Gholami, H.; Ashtekar, K. D.; Acho, R.; Borhan, B. J. Am. Chem. Soc. 2014, 136, 550.
[58] Tanasova, M.; Anyika, M.; Borhan, B. Angew. Chem., Int. Ed. 2015, 54, 4274.
[59] Gholami, H.; Zhang, J.; Anyika, M.; Borhan, B. Org. Lett. 2017, 19, 1722.
[60] Wolf, C.; Bentley, K. W. Chem. Soc. Rev. 2013, 42, 5408.
[61] Zor, E.; Bekar, N. Biosens. Bioelectron. 2017, 91, 211.
[62] Song, G.; Zhou, F.; Xu, C.; Li, B. Analyst 2016, 141, 1257.
[63] Tashkhourian, J.; Afsharinejad, M.; Zolghadr, A. R. Sens. Actuators, B 2016, 232, 52.
[64] Jafari, M.; Tashkhourian, J.; Absalan, G. Talanta 2018, 178, 870.
[65] Kubo, Y.; Maeda, S. Y.; Tokita, S.; Kubo, M. Nature 1996, 382, 522.
[66] Wei, J.; Guo, Y.; Li, J.; Yuan, M.; Long, T.; Liu, Z. Anal. Chem. 2017, 89, 9781.
[67] Fan, C.; Huang, X.; Han, L.; Lu, Z.; Wang, Z.; Yi, Y. Sens. Actuators, B 2016, 224, 592.
[68] Zhou, Y.; Zhang, J. F.; Yoon, J. Chem. Rev. 2014, 114, 5511.
[69] Jung, H. S.; Chen, X.; Kim, J. S.; Yoon, J. Chem. Soc. Rev. 2013, 42, 6019.
[70] Lesot, P.; Aroulanda, C.; Zimmermann, H.; Luz, Z. Chem. Soc. Rev. 2015, 44, 2330.
[71] Plotka, J. M.; Biziuk, M.; Morrison, C. TrAC, Trends Anal. Chem. 2011, 30, 1139.
[72] Aizawa, S. I.; Kidani, T.; Takada, S.; Ofusa, Y. Chirality 2015, 27, 353.
[73] Pirkle, W. H.; Hoover, D. J. Top. Stereochem. 1982, 13, 263.
[74] Silva, M. S. Molecules 2017, 22, 247.
[75] Bian, G.; Yang, S.; Huang, H.; Zong, H.; Song, L.; Fan, H.; Sun, X. Chem. Sci. 2016, 7, 932.
[76] Labuta, J.; Hill, J. P.; Ishihara, S.; Hanykova, L.; Ariga, K. Acc. Chem. Res. 2015, 48, 521.
[77] Zhao, Y.; Swager, T. M. J. Am. Chem. Soc. 2015, 137, 3221.
[78] Yu, X.; Yao, Z.-P. Anal. Chim. Acta 2017, 968, 1.
/
| 〈 |
|
〉 |