

N-苯基吡唑脲类FAK II型抑制剂的结构优化与构效关系研究
收稿日期: 2018-05-08
修回日期: 2018-06-07
网络出版日期: 2018-06-29
基金资助
上海市科委生物医药支撑(No.14431900600)及复旦大学药学院融合基金(No.RO-MY201708)资助项目.
Structure Optimization and Structure-Activity Relationship Study of a Kind of Type II FAK Inhibitors with N-Phenylpyrazole Ureas
Received date: 2018-05-08
Revised date: 2018-06-07
Online published: 2018-06-29
Supported by
Project supported by the Shanghai Science and Technology Commission Support Project for Biological Medicine (No. 14431900600) and the School of Pharmacy of Fudan University Fusion Fund (No. RO-MY201708).
龚超超 , 谈寒一 , 张倩 . N-苯基吡唑脲类FAK II型抑制剂的结构优化与构效关系研究[J]. 有机化学, 2018 , 38(11) : 3086 -3093 . DOI: 10.6023/cjoc201805020
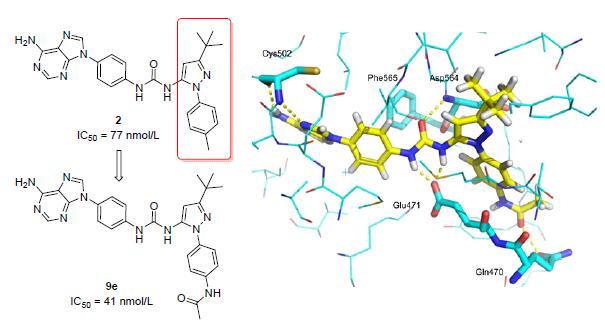
The structure modification and optimization focused on the N-phenylpyrazole motif of the lead compound 2 were conducted on basis of the structural features of focal adhesion kinase (FAK) allosteric hydrophobic pockets. Nine aimed compounds were designed and synthesized, among which four compounds maintained the inhibitory activity against FAK at the same level as 2. Especially, N-(4-(5-(3-(4-(6-amino-9H-purin-9-yl) phenyl) ureido)-3-(tert-butyl)-1H-pyrazol-1-yl) phenyl) ace-tamide (9e) demonstrated 2-fold higher inhibition potency than that of the lead compound with the IC50 value of 41 nmol/L. It is suggested that the bioactivity could be further improved by introducing more proper groups at the 4-position of phenyl to increase the interactions between the substituents and the residues around them.
[1] Zhao, X.; Guan, J. Adv. Drug Delivery Rev. 2011, 63, 610.
[2] Heinrich, T.; Seenisamy, J.; Emmanuvel, L.; Kulkarni, S. S.; Bomke, J.; Rohdich, F.; Greiner, H.; Esdar, C.; Krier, M.; Gradler, U.; Musilet, D. J. Med. Chem. 2013, 56, 1160.
[3] Parsons, J. T. J. Cell Sci. 2003, 116, 1409.
[4] Sulzmaier, F. J.; Jean, C.; Schlaepfer, D. D. Nat. Rev. Cancer 2014, 14, 598.
[5] Schneider, G.; Geppert, T.; Hartenfeller, M.; Reisen, F.; Klenner, A.; Reutlinger, M.; Hähnke, V.; Hiss, J. A.; Zettl, H.; Keppner, S.; Spänkuch, B.; Schneideret, P. Future Med. Chem. 2011, 3, 415.
[6] Peng, W.; Zhang, X. W.; Zhang, C.; Wang, F.; You, Q. D. Chin. J. New Drug 2012, 21, 890(in Chinese). (彭文, 张小猛, 张仓, 王芳, 尤启冬, 中国新药杂志, 2012, 21, 890.)
[7] Dietrich, J.; Hulme, C.; Hurley, L. H. Bioorg. Med. Chem. 2010, 18, 5738.
[8] Infante, J. R.; Camidge, D. R.; Mileshkin, L. R.; Chen, E. X.; Hicks, R. J.; Rischin, D.; Fingert, H.; Pierce, K. J.; Xu, H. P.; Roberts, W. G.; Shreeve, S. M.; Burris, H. A.; Siu, L. L. J. Clin. Oncol. 2012, 30, 1527.
[9] Zhang, J.; He, D. H.; Zajac-Kaye, M.; Hochwald, S. N. Cell Cycle 2014, 13, 3143.
[10] Ott, G. R.; Cheng, M.; Learn, K. S.; Wagner, J.; Gingrich, D. E.; Lisko, J. G.; Curry, M.; Mesaros, E. F.; Ghose, A. K.; Quail, M. R.; Wan, W. H.; Lu, L. H.; Dobrzanski, P.; Albom, M. S.; Angeles, T. S.; Wells-Knecht, K.; Huang, Z. Q.; Aimone, L. D.; Bruckheimer, E.; Anderson, N.; Friedman, J.; Fernandez, S. V.; Ator, M. A.; Ruggeri, B. A.; Dorsey, B. D. J. Med. Chem. 2016, 59, 7478.
[11] Tanjoni, I.; Colin Walsh, C.; Uryu, S.; Tomar, A.; Nam, J.; Mielgo, A.; Lim, S.; Liang, C. X.; Koenig, M.; Patel, N.; Kwok, C.; McMahon, G.; Stupack, D. G.; Schlaepfer, D. D. Cancer Biol. Ther. 2010, 9, 764.
[12] Kang, Y.; Hu, W.; Ivan, C.; Dalton, H. J.; Miyake, T.; Pecot, C. V.; Zand, B.; Liu, T.; Huang, J.; Jennings, N. B.; Rupaimoole, R.; Taylor, M.; Pradeep, S.; Wu, S. Y.; Lu, C. H.; Wen, Y. F.; Huang, J. F.; Liu, J. S.; Sood A. K. J. Natl. Cancer Inst. 2013, 105, 1485.
[13] Iwatani, M.; Iwata, H.; Okabe, A.; Skene, R. J.; Tomita, N.; Hayashi, Y.; Aramaki, Y.; Hosfield, D. J.; Hori, A.; Baba, A.; Miki, H. Eur. J. Med. Chem. 2013, 61, 49.
[14] Johnson, L. N.; Noble, M.; Owen, D. J. Cell 1996, 85, 149.
[15] Grädler, U.; Bomke, J.; Musil, D.; Dresing, V.; Lehmann, M.; Hölzemann, G.; Greiner, H.; Esdar, C.; Krier, M.; Heinrich, T. Bioorg. Med. Chem. Lett. 2013, 23, 5401.
[16] Váňa, L.; Vrzal, L.; DvoRáková, H.; Himl, M.; Linhart, I. Synth. Commun. 2014, 44, 788.
[17] Simay, A.; Takacs, K.; Horvath, K.; Dvortsak, P. Acta Chim. Acad. Sci. Hung. 1980, 105, 127.
/
| 〈 |
|
〉 |