

膦酰保护基促进选择性Hay偶联制备非对称1,3-二炔
收稿日期: 2018-05-02
修回日期: 2018-05-30
网络出版日期: 2018-07-05
基金资助
国家自然科学基金(No.21402048)、湖南省自然科学基金(No.2018JJ3145)、湖南省教育厅一般项目(No.17C0629)、湖南科技大学博士启动基金(No.E51693)资助项目.
Phosphoryl Protecting Group Enabled Facile Synthesis of Unsymmetrical 1,3-Diynes by Selective Hay Coupling
Received date: 2018-05-02
Revised date: 2018-05-30
Online published: 2018-07-05
Supported by
Project supported by the National Natural Science Foundation of China (No. 21402048), the Natural Science Fund Youth Project of Hunan Province (No. 2018JJ3145), the General Project of Hunan Education Department (No. 17C0629) and the Doctoral Foundation of Hunan University of Science and Technology (No. E51693).
彭丽芬 , 彭超 , 汪明 , 唐子龙 , 焦银春 , 许新华 . 膦酰保护基促进选择性Hay偶联制备非对称1,3-二炔[J]. 有机化学, 2018 , 38(11) : 3048 -3055 . DOI: 10.6023/cjoc201805009
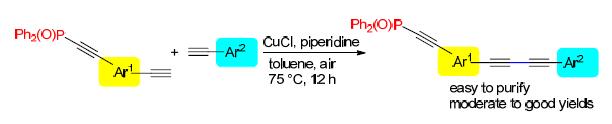
A selective Hay coupling reaction of aromatic terminal acetylenes and monophosphoryl-protected diynes was developed. The polarity of Ph2P(O) realized facile isolation of the desired unsymmetrical 1,3-diynes from by-products. The low reactivity of monophosphoryl-protected diynes reduced the oxidative homocoupling of itself and enhanced the yields of desired products. A number of aromatic terminal acetylenes and monophosphoryl-protected diynes were tolerated in this reaction, and all the corresponding unsymmetrical 1,3-diynes could be obtained in moderate to good yields. The unsymmetrical 1,3-diynes could be applied to synthesize unsymmetrical yne-diynes and cyclic polyynes.
[1] Stang, P. J.; Diederich, F. Modern Acetylene Chemistry, VCH, Weinheim, 1995.
[2] Shi, W.; Lei, A.-W. Tetrahedron Lett. 2014, 55, 2763.
[3] (a) Ito, A.; Cui, B.; Chavez, D.; Chai, H. B.; Shin,Y. G.; Kawanishi, K.; Kardono, L. B.; Riswan, S.; Farnsworth, N. R.; Cordell, G. A.; Pezzuto, J. M.; Kinghorn, A. D. J. Nat. Prod. 2001, 64, 246.
(b) Evano, G.; Blanchard, N. Copper-Mediated Cross-Coupling Reactions, John Wiley & Sons, Inc., Hoboken, NJ, 2014.
(c) Siemsen, P.; Livingston, R. C.; Diederich, F. Angew. Chem. Int. Ed. 2000, 39, 2632.
[4] Guo, L.; Song, L.; Wang, Z.; Zhao, W.; Mao, W.; Yin, M. Chem. Biol. Interact. 2009, 181, 138.
[5] (a) Katano, M.; Yamamoto, H.; Matsunaga, H.; Mori, M.; Takata, K.; Nakamura, M. Gan to Kagaku Ryoho 1990, 17, 1045.
(b) Matsunaga, H.; Saita, T.; Naguo, F.; Mori, M.; Katano, M. Cancer Chemother. Pharmacol. 1995, 35, 291.
[6] (a) Yadav, J. S.; Kumaraswamy, B.; Sathish Reddy, A.; Srihari, P.; Janakiram, R. V.; Kalivendi, S. V. J. Org. Chem. 2011, 76, 2568.
(b) Srihari, P.; Sathish Reddy, A.; Deepthi, Y.; Kalivendi, S. Tetrahedron Lett. 2013, 54, 5616.
[7] Gangadhar, P.; Reddy, S. A.; Srihari, P. Tetrahedron 2016, 72, 5807.
[8] (a) Fang, J.-K.; Sun, T.-X.; Tian, Y.; Zhang, Y.-J.; Jin, C.-F.; Xu, Z.-M.; Hu, X.-Y.; Wang, H.-B. Mater. Chem. Phys. 2017, 195, 1.
(b) Peng, L.-F.; Wang, B.-H.; Wang, M.; Tang, Z.-L.; Jiang, Y.-Z.; Jiao, Y.-C.; Xu, X.-H. J. Chem. Res. 2018, 42, 235.
(c) Peng, L.-F.; Lei, J.-Y.; Wu, L.; Tang, Z.-L.; Luo, Z.-P.; Jiao, Y.-C.; Xu, X.-H. J. Chem. Res. 2018, 42, 271.
[9] Pati, A. K.; Mohapatra, M.; Ghosh, P.; Gharpure, S. J.; Mishra, A. K. J. Phys. Chem. A 2013, 117, 6548.
[10] (a) Wan,W. B.; Brand, S. C.; Pak, J. J.; Haley, M. M. Chem.-Eur. J. 2005, 6, 2044.
(b) Peng, L.-F.; Jiang, J.; Peng, C.; Dai, N.-N.; Tang, Z.-L.; Jiao, Y.-C.; Chen, J.-Y.; Xu, X.-H. Chin. J. Org. Chem. 2017, 37, 3013(in Chinese). (彭丽芬, 蒋娟, 彭超, 代宁宁, 唐子龙, 焦银春, 陈锦杨, 许新华, 有机化学, 2017, 37, 3013.)
(c) Peng, L.-F.; Zhang, S.-W.; Wang, B.-H.; Xun, M.-S.; Tang, Z.-L.; Jiao, Y.-C.; Xu, X.-H. Chin. J. Org. Chem. 2018, 38, 519(in Chinese). (彭丽芬, 张思维, 王丙昊, 寻梦硕, 唐子龙, 焦银春, 许新华, 有机化学, 2018, 38, 519.)
[11] (a) Li, Y.-N.; Wang, J.-L.; He, L.-N. Tetrahedron Lett. 2011, 52, 3485.
(b) Yadav, J. S.; Reddy, B. V. S.; Reddy, K. B.; Gayathri, K. U.; Prasad, A. R. Tetrahedron Lett. 2003, 44, 6493.
[12] (a) Hay, A. J. Org. Chem. 1960, 25, 1275.
(b) Abe, H.; Kurokawa, H.; Chida, Y.; Inouye, M. J. Org. Chem. 2011, 76, 309.
[13] (a) Hay, A. S. J. Org. Chem. 1962, 27, 3320.
(b) Montierth, J. M.; DeMario, D. R.; Kurth, M. J.; Schore, N. E. Tetrahedron 1998, 54, 11741.
[14] (a) Bandyopadhyay, A.; Varghese, B.; Sankararaman, S. J. Org. Chem. 2006, 71, 4544.
(b) Cahiez, G.; Moyeux, A. Chem. Rev. 2010, 110, 1435.
[15] (a) Allen, S. E.; Walvoord, R. R.; Padilla-Salinas, R.; Kozlowski, M. C. Chem. Rev. 2013, 113, 6234.
(b) Siemsen, P.; Livingston, R. C.; Diederich, F. Angew. Chem., Int. Ed. 2000, 39, 2632.
(c) Stefani, H. A.; Guarezemini, A. S.; Cella, R. Tetrahedron 2010, 66, 7871.
(d) Jia, X.; Yin, K.; Li, C.; Li, J.; Bian, H. Green Chem. 2011, 13, 2175.
(e) Kamata, K.; Yamaguchi, S.; Kotani, M.; Yamaguchi, K.; Mizuno, N. Angew. Chem., Int. Ed. 2008, 47, 2407.
(f) Crowley, J. D.; Goldup, S. M.; Gowans, N. D.; Leigh, D. A.; Ronaldson, V. E.; Slawin, A. M. Z. J. Am. Chem. Soc. 2010, 132, 6243.
(g) Gao, H.-Y.; Wagner, H.; Zhong, D.; Franke, J.-H.; Studer, A.; Fuchs, H. Angew. Chem., Int. Ed. 2013, 52, 4024.
(h) Zhang, S.; Liu, X.; Wang, T. Adv. Synth. Catal. 2011, 353, 1463.
(i) Balamurugan, R.; Naveen, N.; Manojveer, S.; Nama, M. V. Aust. J. Chem. 2011, 64, 567.
(j) Wong, W.-Y.; Lu, G.-L.; Choi, K.-H.; Guo, Y.-H. J. Organomet. Chem. 2005, 690, 177.
(k) Navale, B. S.; Bhat, R. G. RSC Adv. 2013, 3, 5220.
(l) Liu, Y.; Wang, C.; Wang, X.; Wan, J.-P. Tetrahedron Lett. 2013, 54, 3953.
[16] (a) Yin, W.; He, C.; Chen, M.; Zhang, H.; Lei, A. Org. Lett. 2009, 11, 709.
(b) Suarez, J. R.; Collado-Sanz, D.; Cardenas, D. J.; Chiara, J. L. J. Org. Chem. 2015, 80, 1098.
(c) Balaraman, K.; Kesavan, V. Synthesis 2010, 3461.
(d) Lampkowski, J. S.; Durham, C. E.; Padilla, M. S.; Young, D. D. Org. Biomol. Chem. 2015, 13, 424.
[17] (a) Cadiot, P.; Chodkiewicz, W. Chemistry of Acetylenes, Marcel Dekker, New York, 1969.
(b) Sindhu, K. S.; Thankachan, A. P.; Sajitha, P. S.; Anilkumar, G. Org. Biomol. Chem. 2015, 13, 6891.
(c) Yu, M.; Pan, D.; Jia, W.; Chen, W.; Jiao, N. Tetrahedron Lett. 2010, 51, 1287.
[18] (a) Peng, H.-H.; Xi, Y.-M.; Ronaghi, N.; Dong, B.-L.; Akhmedov, N. G.; Shi, X.-D. J. Am. Chem. Soc. 2014, 136, 13174.
(b) Vilhanová, B.; Václavík, J.; Artiglia, L.; Ranocchiari, M.; Togni, A.; Bokhoven, J. A. ACS Catal. 2017, 7, 3414.
[19] Lampkowski, J. S.; Uthappa, D. M.; Halonski, J. F.; Maza, J. C.; Young, D. D. J. Org. Chem. 2016, 81, 12520.
[20] Su, L.-B.; Dong, J.-Y.; Liu, L.; Sun, M.-L.; Qiu, R.-H.; Zhou, Y.-B.; Yin, S.-F. J. Am. Chem. Soc. 2016, 138, 12348.
[21] Wan, J.-P.; Cao, S.; Jing, Y.-F. Appl. Organomet. Chem. 2014, 28, 631.
[22] Yang, X.; Matsuo, D.; Suzuma, Y.; Fang, J.-K.; Xu, F.; Orita, A.; Otera, J. Synlett 2011, 2402.
[23] Peng, L.-F.; Xu, F.; Suzuma, Y.; Orita, A.; Otera, J. J. Org. Chem. 2013, 78, 12802.
[24] Peng, L.-F.; Xu, F.; Shinohara, K.; Orita, A.; Otera, J. Chem. Lett. 2014, 43, 1610.
[25] Peng, L.-F.; Xu, F.; Shinohara, K.; Orita, A.; Otera, J. Org. Chem. Front. 2015, 2, 248.
/
| 〈 |
|
〉 |