

2-甲硫基-4-芳胺喹唑啉衍生物的合成及抗肿瘤活性评价
收稿日期: 2018-05-04
修回日期: 2018-06-20
网络出版日期: 2018-07-05
基金资助
国家自然科学基金(No.81430085)、河南省科技厅项目(Nos.182300410321,182102310249)和郑州大学大学生创新创业训练计划(No.2017cxcy563)资助项目.
Synthesis and Antitumor Activities Evaluation of 2-Methylthio-4-arylamine Quinazoline Derivatives
Received date: 2018-05-04
Revised date: 2018-06-20
Online published: 2018-07-05
Supported by
Project supported by the National Natural Science Foundation of China (No. 81430085), the Technology Department Project of Henan Province (Nos. 182300410321, 182102310249) and the Students' Platform for Innovation and Entrepreneurship Training Program of Zhengzhou University (No. 2017-cxcy563).
孟祥川 , 栗娜 , 李二冬 , 常通航 , 刘梦 , 柳晴怡 , 袁文娟 , 张晴晴 , 张钰 , 周智玉 , 宋攀攀 , 刘宏民 , 张秋荣 . 2-甲硫基-4-芳胺喹唑啉衍生物的合成及抗肿瘤活性评价[J]. 有机化学, 2018 , 38(11) : 3063 -3069 . DOI: 10.6023/cjoc201805015
In order to find the efficient and novel antitumor drugs, a series of 2-methylthio-4-arylamine quinazoline derivatives were designed and synthesized. The target compounds were evaluated for antitumor activity in vitro on four human cancer cell lines initially including MGC-803, PC-3, HGC-27 and A549. The results showed that some compounds had good antitumor activities, especially N-(4-fluorophenyl)-2-(methylthio) quinazolin-4-amine (9c) and 2-(methylthio)-N-(p-tolyl)-quina-zolin-4-amine (9m), with IC50 values of 0.18 and 0.68 μmol·L-1, respectively.
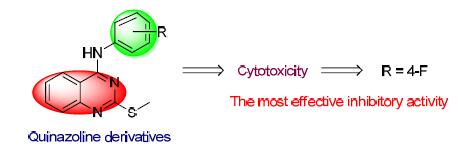
Key words: quinazoline derivatives; synthesis; antitumor activity; evaluation
[1] Sangthong, S.; Krusong, K.; Ngamrojanavanich, N.; Vilaivan, T.; Puthong, S.; Chandchawan, S.; Muangsin, N. Bioorg. Med. Chem. Lett. 2011, 21, 4813.
[2] Cao, R. H.; Fan, W. X.; Guo, L.; Ma, Q.; Zhang, G. X.; Li, J. R.; Chen, X. M.; Ren, Z. H.; Qiu, L. Q. Eur. J. Med. Chem. 2013, 60, 135.
[3] Van Horn, K. S.; Burda, W. N.; Fleeman, R.; Shaw, L. N.; Manetsch, R. J. Med. Chem. 2014, 57, 3075.
[4] Liu, F.; Huang, Y. J. Pestic. Biochem. Phys. 2011, 101, 248.
[5] Alafeefy, A. M.; Kadi, A. A.; Al-Deeb, O. A.; El-Tahir, K. E. H.; Al-Jaber, N. A. Eur. J. Med. Chem. 2010, 45, 4947.
[6] Wu, J. W.; Chen, W. T.; Xia, G. X.; Zhang, J.; Shao, J. A.; Tan, B. Q.; Zhang, Z. Z.; Yu, W. W.; Weng, Q. J.; Liu, H. Y.; Hu, M.; Deng, H. L.; Hao, Y.; Shen, J. K.; Yu, Y. P. ACS Med. Chem. Lett. 2013, 4, 974.
[7] Zhang, Y. L.; Chen, L.; Xu, H. J.; Li, X. B.; Zhao, L. J.; Wang, W.; Li, B. L.; Zhang, X. Q. Eur. J. Med. Chem. 2018, 147, 77.
[8] Siegel, R.; Naishadham, D.; Jemal, A. CA Cancer J. Clin. 2013, 67, 11.
[9] Cui, G.; Cui, M.; Li, Y.; Liang, Y.; Li, W.; Guo, H.; Zha, S. Med. Oncol. 2015, 32, 570.
[10] Mathew, M. P.; Tan, E.; Saeui, C. T.; Bovonratwet, P.; Liu, L.; Bhattacharya, R.; Yarema, K. J. Bioorg. Med. Chem. Lett. 2015, 25, 1223.
[11] Smaill, J. B.; Showalter, H. D. H.; Zhou, H.; Bridges, A. J.; McNamara, D. J.; Fry, D. W.; Nelson, J. M.; Sherwood, V.; Vincent, P. W.; Roberts, B. J.; Elliott, W. L.; Denny, W. A. J. Med. Chem. 2001, 44, 429.
[12] Engelman, J. A.; Zejnullahu, F.; Gale, C.-M.; Lifshits, E.; Gonzales, A. J.; Shimamura, T.; Zhao, F.; Vincent, P. W.; Naumov, G. N.; Bradner, J. E.; Althaus, I. W.; Gandhi, L.; Shapiro, G. I.; Nelson, J. M.; Heymach, J. V.; Meyerson, M.; Wong, K. K.; Janne, P. A. Cancer Res. 2007, 67, 11924.
[13] Li, D.; Ambrogio, L.; Shimamura, T.; Kubo, S.; Takahashi, M.; Chirieac, L. R.; Padera, R. F.; Shapiro, G. I.; Baum, A.; Himmelsbach, F.; Rettig, W. J.; Meyerson, M.; Solca, F.; Greulich, H.; Wong, K. K. Oncogene 2008, 27, 4702.
[14] Al-Rashood, S. T.; Aboldahab, I. A.; Nagi, M. N.; Abouzeid, L. A.; Abdel-Aziz, A. A. M.; Abdel-Hamide, S. G.; Youssef, K. M.; Al-Obaid, A. M.; El-Subbagh, H. I. Bioorg. Med. Chem. 2006, 14, 8608.
[15] Shao, K. P.; Zhang, X. Y.; Chen, P. J.; Xue, D. Q.; He, P.; Ma, L. Y.; Zheng, J. X.; Zhang, Q. R.; Liu, H. M. Bioorg. Med. Chem. Lett. 2014, 24, 3877.
[16] Wang, C.; Cao, Q.; Yang, H.; Song, P.; Xue, D.; Cui, F.; Gu, Y.; Zhang, X.; Tian, Y.; Zhang, Q.; Liu, H. Chin. J. Org. Chem. 2016, 36, 1626(in Chinese). (王超杰, 曹钦坡, 杨慧, 宋攀攀, 薛登启, 崔飞, 顾一飞, 张孝松, 田亚楠, 张秋荣, 刘宏民, 有机化学, 2016, 36, 1626.)
[17] Li, N.; Xin, J. C.; Ma, Q. S.; Li, E. D.; Meng, Y. Q.; Bao, C. N.; Yang, P.; Song, P. P.; Cui, F.; Cheng, P. J.; Gu, Y. F.; Zhao, P. R.; Ke, Y.; Liu, H. M.; Zhang, Q. R. Chin. J. Org. Chem. 2018, 38, 665(in Chinese). (栗娜, 辛景超, 马启胜, 李二冬, 孟娅琪, 包崇男, 杨鹏, 宋攀攀, 崔飞, 陈鹏举, 顾一飞, 赵培荣, 可钰, 刘宏民, 张秋荣, 有机化学, 2018, 38, 665.)
[18] Xin, J. C.; Li, N.; Ma, Q. S.; Li, E. D.; Meng, X. C.; Ke, Y.; Liu, H. M.; Zhang, Q. R. Chin. J. Org. Chem. 2018, 32, 451(in Chinese). (辛景超, 栗娜, 马启胜, 李二冬, 孟祥川, 可钰, 刘宏民, 张秋荣, 有机化学, 2018, 32, 451.)
[19] Abdullaev, N. P.; Kayumov, K.; Shakhidoyatov, K. M. Uzb. Khim. Zh. 1997, 2, 29.
[20] Kikelj, D. In Science of Synthesis, Product Class 13:Quinazolines, Vol. 16, Eds.:Yamamoto, Y.; Bellus, D.; Jacobsen, E. N.; Ley, S. V.; Noyori, R.; Regitz, M.; Reider, P. J.; Schaumann, E.; Shinkai, I.; Thimas, E. J.; Trost, B. M., Thieme, Stuttgart, 2004, p. 573.
[21] Song, P. P.; Cui, F.; Li, N.; Xin, J. C.; Ma, Q. S.; Meng, X. C.; Wang, C. J.; Cao, Q. P.; Gu, Y. F.; Ke, Y.; Zhang, Q. R.; Liu, H. M. Chin. J. Chem. 2017, 35, 1633(in Chinese). (宋攀攀, 崔飞, 栗娜, 辛景超, 马启胜, 孟祥川, 王超杰, 曹钦坡, 顾一飞, 可钰, 刘宏民, 张秋荣, 有机化学, 2017, 35, 1633.)
[22] Shao, K. P.; Zhang, X. Yao.; Chen, P. J.; Xue, D. Q.; He, P.; Ma, L. Y.; Zheng, J. X.; Zhang, Q. R.; Liu, H. M. Bioorg. Med. Chem. Lett. 2014, 24, 3877.
[23] Chen, P. J.; Yang, A.; Gu, Y. F.; Zhang, X. S.; Shao, K. P.; Xue, D. Q.; He, P.; Jiang, T. F.; Zhang, Q. R.; Liu, H. M. Bioorg. Med. Chem. Lett. 2014, 24, 2741.
/
| 〈 |
|
〉 |