

手性类卟啉N4配体与锰络合物氧化动力学拆分消旋芳香环亚砜
收稿日期: 2018-05-16
修回日期: 2018-06-19
网络出版日期: 2018-07-05
基金资助
国家自然科学基金(No.21403100)、辽宁省教育厅高等教育研究基金(No.L2014421)资助项目.
Oxidation Kinetics Resolution of Racemic Aromatic Sulfoxides by Chiral Porphyrin-Inspired N4 Ligand with Manganese Complex
Received date: 2018-05-16
Revised date: 2018-06-19
Online published: 2018-07-05
Supported by
Project supported by the National Natural Science Foundation of China (No. 21403100) and the Education Research Program of the Education Department of Liaoning Province (No. L2014421).
杨金闯, 李国松, 吕成伟, 安悦, 高爽 . 手性类卟啉N4配体与锰络合物氧化动力学拆分消旋芳香环亚砜[J]. 有机化学, 2018 , 38(11) : 3070 -3077 . DOI: 10.6023/cjoc201805034
Oxidative kinetics resolution of racemic aromatic sulfoxide was studied by using chiral porphyrin-inspired N4 ligands and manganese in situ complex as catalyst, environment-friendly H2O2 as oxidant and adamantanecarboxylic acid as additive. The arylalkyl and arylbenzyl sulfoxide substrates were extended by this catalytic system. A maximum yield of chiral sulfoxide was 40% and the enantioselectivity was 100%. In the meantime, the yield of sulfone a further oxidation products of sulfoxide, was up to 72%. It was found that the catalytic oxidation system is more prone to electron-rich sulfoxide through the competition experiment between electron-rich sulfoxide and electron-deficient sulfoxide substrates. In addition, the success of gram-scale oxidation kinetic resolution also shows that this method has a certain practical value in methodology.
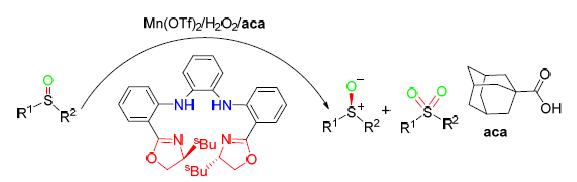
Key words: ligand N4; manganese; oxidative kinetic resolution; aromatic sulfoxides; sulfone
[1] (a) Kielbasiński, P.; Mikolajczyk, M.; Zwanenburg, B.; De Laet, R. C. Phosphorus, Sulfur, Silicon Related Elem. 1994, 95, 495.
(b) Kwiatkowska, M.; Janicki, I.; Kielbasiński, P. J. Mol. Catal. B:Enzym. 2015, 118, 23.
[2] Pitchen, P.; Dunach, E.; Deshmukh, M.; Kagan, H. J. Am. Chem. Soc. 1984, 106, 8188.
[3] (a) Tang, J.; Yao, P. F.; Xu, X. L.; Li, H. Y.; Huang, F. P.; Nie, Q. Q.; Luo, M. Y.; Yu, Q.; Bian, H. D. RSC Adv. 2016, 6, 44154.
(b) Romanowski, G.; Kira, J. Polyhedron 2016, 117, 352.
(c) Jahier, C.; Touzani, R.; Kadiri, S. E.; Nlate, S. Inorg. Chim. Acta 2016, 450, 81.
(d) Zeng, Q. L. Prog Chem. 2007, 19, 745(in Chinese) (曾庆乐, 化学进展, 2007, 19, 745.)
[4] Jiang, B.; Huang, H.; Luo, J.; Li, Z. Y. Chin. J. Org. Chem. 2005, 25, 1542(in Chinese). (姜标, 黄浩, 罗军, 李祖义, 有机化学, 2005, 25, 1542.)
[5] (a) Wojaczyńska, E.; Wojaczyński, J. Chem. Rev. 2010, 110, 4303.
(b) Chen, K. Q.; Li, K. Chin. J. Org. Chem. 1989, 9, 8(in Chinese). (陈克潜; 李凯. 有机化学, 1989, 9, 8.)
[6] (a) Fernández, I; Khiar, N. Chem. Rev. 2003, 103, 3651.
(b) Adrio, J.; Carretero, J. C. J. Am. Chem. Soc. 1999, 121, 7411.
(c) Chen, M. S.; Prabagaran, N.; Labenz, N. A.; White, M. C. J. Am. Chem. Soc. 2005, 127, 6970.
(d) Mariz, R.; Luan, X.; Gatti, M.; Linden, A.; Dorta, R. J. Am. Chem. Soc. 2008, 130, 2172.
(e) Bürgi, J. J.; Mariz, R.; Gatti, M.; Drinkel, E.; Luan, X.; Blumentritt, S.; Linden, A.; Dorta, R. Angew. Chem., Int. Ed. 2009, 48, 2768.
(f) Carreno, M. C.; Des Mazery, R.; Urbano, A.; Colobert, F.; Solladié, G. Org. Lett. 2004, 6, 297.
[7] (a) Di Furia, F.; Modena, G.; Seraglia, R. Synthesis 1984, 325.
(b) Furia, F. D.; Modene, G.; Seraglia, R. Synthesis 1984, 15, 325.
(c) Ligtenbarg, A. G.; Hage, R.; Feringa, B. L. Coord. Chem. Rev. 2003, 237, 89.
[8] (a) Sturala, J.; Bohacova, S.; Chudoba, J.; Metelkova, R.; Cibulka, R. J. Org. Chem. 2015, 80, 2676.
(b) Hassanpour, A.; Acuña-Parés, F.; Luis, J. M.; Cusso, O.; Morales, d. l. R. S.; Campos-Martín, J. M.; Fierro, J. L.; Costas, M.; Lloret-Fillol, J.; Mas-Ballesté, R. Chem. Commun. 2015, 51, 14992.
(c) Leow, W. R.; Ng, W. K.; Peng, T.; Liu, X.; Li, B.; Shi, W.; Lum, Y.; Wang, X.; Lang, X.; Li, S. J. Am. Chem. Soc. 2016.
(d) Zhao, L.; Zhang, H.; Wang, Y. J. Org. Chem. 2016, 81, 129.
(e) Chatterjee, S.; Paine, T. K. Angew. Chem. 2016, 128, 7848.
[9] (a) Rioz-Martínez, A.; de Gonzalo, G.; Pazmiño, D. E. T.; Fraaije, M. W.; Gotor, V. Eur. J. Org. Chem. 2010, 6409.
(b) Drago, C.; Caggiano, L.; Jackson, R. F. Angew. Chem. 2005, 117, 7387.
(c) Boruah, J. J.; Ahmed, K.; Das, S.; Gogoi, S. R.; Saikia, G.; Sharma, M.; Islam, N. S. Mol. Catal. A:Chem. 2016, 425, 21.
[10] (a) Xu, L.; Cheng, J.; Trudell, M. L. J. Org. Chem. 2003, 68, 5388.
(b) Fukuda, N.; Ikemoto, T. J. Org. Chem. 2010, 75, 4629.
(c) Zhao, W.; Yang, C.; Cheng, Z.; Zhang, Z. Green Chem. 2016, 18, 995.
(d) Fareghi-Alamdari, R.; Zekri, N.; Moghadam, A. J.; Farsani, M. R. Catal. Commun. 2017, 98.
(e) Shyam, P.; Jang, H. Y. J. Org. Chem. 2017, 82.
[11] Bryliakov, K. P.; Talsi, E. P. Eur. J. Org. Chem. 2008, 3369.
[12] Sun, J.; Zhu, C.; Dai, Z.; Yang, M.; Pan, Y.; Hu, H. J. Org. Chem. 2004, 69, 8500.
[13] Bryliakov, K. P.; Talsi, E. P. Chem. Eur. J. 2007, 13, 8045.
[14] Yamaguchi, T.; Matsumoto, K.; Saito, B.; Katsuki, T. Angew. Chem. 2007, 119, 4813.
[15] O'Mahony, G. E.; Ford, A.; Maguire, A. R. J. Org. Chem. 2012, 77, 3288.
[16] (a) Jia, X.; Li, X.; Xu, L.; Li, Y.; Shi, Q.; Au-Yeung, T. T. L.; Yip, C.; Yao, X.; Chan, A. S. Adv. Synth. Catal. 2004, 346, 723.
(b) Zeng, Q.; Wang, H.; Wang, T.; Cai, Y.; Weng, W.; Zhao, Y. Adv. Synth. Catal. 2005, 347, 1933.
[17] Dai, W.; Li, J.; Li, G.; Yang, H.; Wang, L.; Gao, S. Org. Lett. 2013, 15, 4138.
[18] (a) Dai, W.; Li, J.; Chen, B.; Li, G.; Lü, Y.; Wang, L.; Gao, S. Org. Lett. 2013, 15, 5658.
(b) Perrin, D. D.; Armarego, W. L. F.; Perrin, D. R. Purification of Laboratory Chemicals, Pergamon Press, Oxford, 1980.
[19] Yang, J.; Wang, L.; Lv, Y.; Li, N.; An, Y.; Gao, S. Tetrahedron Lett. 2018, 59, 156.
[20] (a) O'Mahony, G. E.; Ford, A.; Maguire, A. R. J. Org. Chem. 2012, 77, 3288.
(b) Le, M. P.; Simonneaux, G. Chem. Comm. 2011, 47, 6957.
(c) Kelly, P.; Lawrence, S. E.; Maguire, A. R. Synlett 2007, 38, 1501.
[21] (a) Voutyritsa, E.; Triandafillidi, I.; Kokotos, C. Synthesis 2016, 49, 917.
(b) Yang, C.; Jin, Q.; Zhang, H.; Liao, J.; Zhu, J.; Yu, B.; Deng, J. Green Chem. 2009, 11, 1401.
(c) Jereb, M. Green Chem. 2012, 44, 3047.
[22] (a) Legros, J.; Bolm, C. Angew. Chem. 2004, 42, 5487.
(b) Legros, J.; Bolm, C. Chemistry 2005, 11, 1086.
(c) Legros, J.; Bolm, C. Angew. Chem. 2003, 115, 5487.
[23] Meckler, H.; Herr, R. J. Org. Process Res. Dev. 2012, 16, 550.
[24] (a) Sonopo, M. S.; Pillay, A.; Chibale, K.; Marjanovic-Painter, B.; Donini, C.; Zeevaart, J. R. J. Labelled Compd. Radiopharm. 2016, 59, 680.
(b) Emrick, D. D.; Truce, W. E. J. Org. Chem. 1960, 25, 1103.
(c) Gilman, H.; Martin, G. A. J. Am. Chem. Soc. 2002, 74, 5317.
[25] Egami, H.; Katsuki, T. J. Am. Chem. Soc. 2007, 129, 8940.
[26] Hanson, P.; Hendrickx, R. A.; Smith, J. R. Org. Biomol. Chem. 2008, 6, 745.
[27] Nodiff, E. A.; Lipschutz, S.; Craig, P. N.; Gordon, M. J. Org. Chem. 1960, 25, 60.
[28] Hanson, P.; Hendrickx, R. A.; Smith, J. R. Org. Biomol. Chem. 2008, 6, 745.
[29] Shaabani, A.; Soleimani, E. Phosphorus, Sulfur Silicon Relat. Elem. 2006, 181, 2475.
[30] Shaabani, A.; Teimouri, F.; Lee, D. G. Synth. Commun. 2003, 33, 1057.
[31] Supale, A. R.; Gokavi, G. S. Phosphorus, Sulfur Silicon Relat. Elem. 2010, 185, 725.
[32] Greger, J. G.; Yoonmiller, S. J. P.; Bechtold, N. R.; Flewelling, S. A.; Macdonald, J. P.; Downey, C. R.; Cohen, E. A.; Pelkey, E. T. J. Org. Chem. 2012, 43, 8203.
[33] Liao, S.; List, B. Adv. Synth. Catal. 2012, 354, 2363.
[34] Ellervik, U.; Jacobsson, M.; Ohlsson, J. Tetrahedron 2005, 61, 2421.
[35] Gogoi, S.; Boruah, J.; Sengupta, G.; Saikia, G.; Ahmed, K.; Bania, K.; Islam, N. Catal. Sci. Technol. 2014, 5, 595.
/
| 〈 |
|
〉 |