

3,4-桥环吲哚类生物碱的合成进展
收稿日期: 2018-05-31
修回日期: 2018-06-25
网络出版日期: 2018-07-05
基金资助
国家自然科学基金(Nos.21572008,21372017)资助项目.
Recent Progress in the Synthesis of 3,4-Fused Indole Alkaloids
Received date: 2018-05-31
Revised date: 2018-06-25
Online published: 2018-07-05
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21572008, 21372017).
袁括 , 贾彦兴 . 3,4-桥环吲哚类生物碱的合成进展[J]. 有机化学, 2018 , 38(9) : 2386 -2399 . DOI: 10.6023/cjoc201705058
3,4-Fused indole alkaloids are an important part of naturally occurring indole alkaloids and have attracted considerable interests from synthetic chemists because of their unique structures and various biological activities. In this review, the recent total syntheses of the 3,4-fused indole alkaloids from 2013 are summerized and classified by the ring-closing positions of the indole 3,4-fused ring.
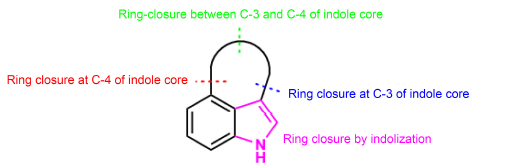
Key words: indole alkaloid; 3,4-fused indole; natural product; total synthesis
[1] Sinz, A. Pharm. Unserer Zeit 2008, 37, 306.
[2] Kamigauchi, T.; Yasui, M. WO 2000059909, 2000.
[3] Riley, R. T.; Goeger, D. E.; Yoo, H.; Showker, J. L. Toxicol. Appl. Pharmacol. 1992, 114, 261.
[4] Moncoq, K.; Trieber, C. A.; Young, H. S. J. Biol. Chem. 2007, 282, 9748.
[5] Hymery, N.; Masson, F.; Barbier, G.; Coton, E. Toxicol. In Vitro 2014, 28, 940.
[6] Shan, D.; Jia, Y. Chin. J. Org. Chem. 2013, 33, 1144(in Chinese). (单冬, 贾彦兴, 有机化学, 2013, 33, 1144.)
[7] McCabe, S. R.; Wipf, P. Org. Biomol. Chem. 2016, 14, 5894.
[8] Liu, H.; Jia, Y. Nat. Prod. Rep. 2017, 34, 411.
[9] Zhang, Y. A.; Liu, Q.; Wang, C.; Jia, Y. Org. Lett. 2013, 15, 3662.
[10] Qin, H.; Xu, Z.; Cui, Y.; Jia, Y. Angew. Chem., Int. Ed. 2011, 50, 4447.
[11] Guo, L.; Zhang, F.; Hu, W.; Li, L.; Jia, Y. Chem. Commun. 2014, 50, 3299.
[12] Zhang, F.; Guo, L.; Hu, W.; Jia, Y. Tetrahedron 2015, 71, 3756.
[13] Liang, K.; Yang, J.; Tong, X.; Shang, W.; Pan, Z.; Xia, C. Org. Lett. 2016, 18, 1474.
[14] Wang, W.; Lu, J. T.; Zhang, H.; Shi, Z.; Wen, J.; Cao, X. J. Org. Chem. 2014, 79, 122.
[15] Netz, N.; Opatz, T. J. Org. Chem. 2016, 81, 1723.
[16] Chen, J.; Song, L.; Li, F.; Shi, Z.; Cao, X. Chem. Commun. 2017, 53, 12902.
[17] Chaudhuri, S.; Ghosh, S.; Bhunia, S.; Bisai, A. Chem. Commun. 2018, 54, 940.
[18] Lu, Z.; Yang, M.; Chen, P.; Xiong, X.; Li, A. Angew. Chem., Int. Ed. 2014, 53, 13840.
[19] Baran, P.; Maimone, T.; Richter, J. Nature 2007446, 404.
[20] Zhou, S.; Chen, H.; Luo, Y.; Zhang, W.; Li, A. Angew. Chem., Int. Ed. 2015, 54, 6878.
[21] Dethe, D. H.; Sau, S. K.; Mahapatra, S. Org. Lett. 2016, 18, 6392.
[22] Dethe, D. H.; Sau, S. K. Org. Lett. 2018, 20, 632.
[23] Lu, Z.; Li, H.; Bian, M.; Li, A. J. Am. Chem. Soc. 2015, 137, 13764.
[24] Zhurakovskyi, O.; Tgrkmen, Y. E.; Löffler, L. E.; Moorthie, V. A.; Chen, C. C.; Shaw, M. A.; Crimmin, M. R.; Ferrara, M.; Ahmad, M.; Ostovar, M.; Matlock, J. V.; Aggarwal, V. K. Angew. Chem., Int. Ed. 2018, 57, 1346.
[25] Lei, T.; Zhang, H.; Yang, Y. R. Tetrahedron Lett. 2015, 56, 5933.
[26] Liang, K.; Wu, T.; Xia, C. Org. Biomol. Chem. 2016, 14, 4690.
[27] Lu, Y.; Yuan, H.; Zhou, S.; Luo, T. Org. Lett. 2017, 19, 620.
[28] Liu, Q.; Li, Q. J.; Ma, Y. F.; Jia, Y. Org. Lett. 2013, 15, 4528.
[29] Lv, J.; Wang, B.; Yuan, K.; Wang, Y.; Jia, Y. Org. Lett. 2017, 19, 3664.
[30] Bhunia, S.; Chaudhuri, S.; Bisai, A. Chem.-Eur. J. 2017, 23, 11234.
[31] Chaudhuri, S.; Bhunia, S.; Roy, A.; Das, M. K.; Bisai, A. Org. Lett. 2018, 20, 288.
[32] Noji, T.; Okano, K.; Tokuyama, H. Tetrahedron 2015, 71, 3833.
[33] Bronner, S. M.; Goetz, A. E.; Garg, N. K. J. Am. Chem. Soc. 2011, 133, 3832.
[34] Nathel, N. F.; Shah, T. K.; Bronner, S. M.; Garg, N. K. Chem. Sci. 2014, 5, 2184.
[35] Miura, T.; Funakoshi, Y.; Murakami, M. J. Am. Chem. Soc. 2014, 136, 2272.
[36] Tao, P.; Jia, Y. Chem. Commun. 2014, 50, 7367.
[37] Zhang, X.; Li, Y.; Shi, H.; Zhang, L.; Zhang, S.; Xu, X.; Liu, Q. Chem. Commun. 2014, 50, 7306.
[38] Zhou, B.; Yang, Y.; Tang, H.; Du, J.; Feng, H.; Li, Y. Org. Lett. 2014, 16, 3900.
[39] Suzuki, Y.; Tanaka, Y.; Nakano, S.; Dodo, K.; Yoda, N.; Shinohara, K.; Kita, K.; Kaneda, A.; Sodeoka, M.; Hamada, Y.; Nemoto, T. Chemistry 2016, 22, 4418.
[40] Suetsugu, S.; Nishiguchi, H.; Tsukano, C.; Takemoto, Y. Org. Lett. 2014, 16, 996.
[41] Park, J.; Kim, D. H.; Das, T.; Cho, C. G. Org. Lett. 2016, 18, 5098.
[42] Yuan, H.; Guo, Z.; Luo, T. Org. Lett. 2017, 19, 624.
[43] Liu, H.; Zhang, X.; Shan, D.; Pitchakuntla, M.; Ma, Y.; Jia, Y. Org. Lett. 2017, 19, 3323.
[44] Zhang, Y.; Hubbard, J. W.; Akhmedov, N. G.; Petersen, J. L.; Söderberg, B. C. J. Org. Chem. 2015, 80, 4783.
[45] Zhang, Y.; McArdle, I. W.; Hubbard, J. W.; Akhmedov, N. G.; Söderberg, B. C. G. Tetrahedron Lett. 2016, 57, 2865.
[46] Tao, P.; Jia, Y. Chem. Commun. 2014, 50, 7367.
[47] Tao, P.; Chen, Z.; Jia, Y. Chem. Commun. 2016, 52, 11300.
[48] McCabe, S. R.; Wipf, P. Angew. Chem., Int. Ed. 2017, 56, 324.
[49] Li, L.; Chen, Z.; Zhang, X.; Jia, Y. Chem. Rev. 2018, 118, 3752.
/
| 〈 |
|
〉 |